Abstract
Guava is a popular subtropical fruit tree grown commercially in south Florida. Production is affected by multiple diseases of different origin. This guide provides information about the most common diseases, including disease biology and management.
This publication targets diagnosticians, growers with basic knowledge of plant diseases, Extension agents, and students. Always follow label instructions. Check pesticide labels for specific host information, target pest and disease, possible phytotoxicity, rates, re-entry intervals, and resistance management information. Some crop protection products may not be registered for sale or use in all states or counties. Check with your state or local Extension service to ensure registration status. Recommendations are based on literature and publicly available databases, but we do not guarantee the efficacy of the active ingredients mentioned in this guide.
Guava Diseases Caused by Fungi and Oomycetes
Anthracnose
Anthracnose is the main disease affecting guava fruit, both pre- and postharvest (Lim and Manicom 2003). This disease affects developing flowers and young fruit, and symptoms appear during fruit ripening, causing considerable postharvest losses. Anthracnose has been reported in all guava-growing areas around the world where high rainfall and humidity are present.
Symptoms
Symptoms of this disease are easily observed on mature fruits. Characteristic symptoms consist of sunken, dark-colored, necrotic lesions. Under humid conditions, the necrotic lesions (dead tissues) become covered with pale-orange or salmon-colored spore masses (conidia). As the disease progresses, the small, sunken lesions coalesce to form large, necrotic patches that affect the pulp of the fruit (Figure 1A–E).
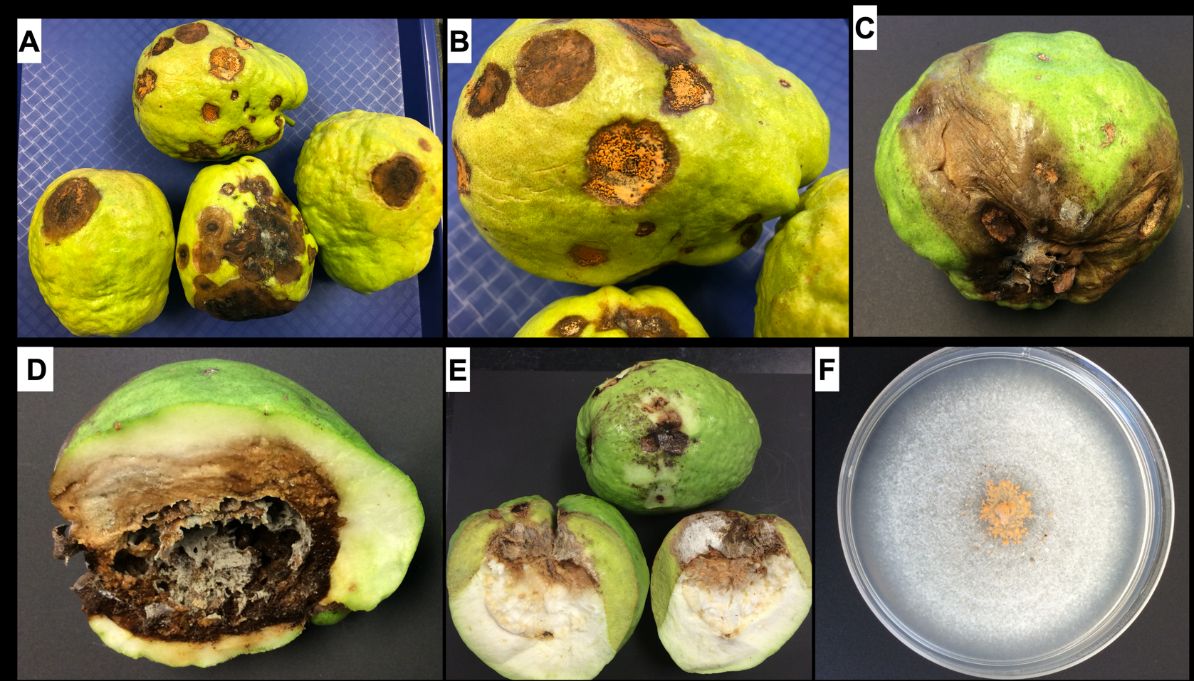
Credit: Romina Gazis, UF/IFAS TREC
Causal Organism
Fungal species in the Colletotrichum gloeosporioides (sexual stage: Glomerella cingulata) complex are common in warm, humid areas and are often generalists (species that can infect multiple hosts, including many tropical fruit crops). Colletotrichum species cause anthracnose symptoms (dark lesions or spots) on fruits, leaves, and flowers. The asexual stage of the fungus is most important in the disease cycle, and the sexual stage plays a role in survival. Colonies of C. gloeosporioides on artificial nutrient media (e.g., potato dextrose agar) are grayish white to dark gray. Production of aerial mycelia by different strains of the fungus varies, ranging from a thick mat to sparse tufts associated with fructification (forming reproductive structures) (Figure 1F). Conidia (asexual spores) are hyaline, unicellular, either cylindrical or ellipsoidal, and contained in a mucilaginous matrix. Conidia are produced on hyaline to faintly brown conidiophores contained in structures called an acervulus (plural: acervuli). These reproductive structures are surrounded by setae (sterile, hairlike fungal structures) which present one to four septa and are brown, slightly swollen at the base, and tapered at the apex.
Disease Cycle and Epidemiology
Infection occurs before harvest. Conidia are responsible for infection. Conidia are produced on dead twigs, inflorescences, and necrotic leaf and fruit lesions. Inflorescences and young fruits are susceptible and, if infected, may abort and abscise. Spores are spread via rain splash and can cause new infections; symptoms may develop shortly thereafter on any aboveground host tissue. Dormant infections (latent infections) are common with this disease, and the pathogen may remain inactive with no symptoms for months (Hossain and Meah 1992). Disease outbreaks can develop during prolonged periods of warm, wet weather, and disease development on uninjured fruits is lower than on injured fruits (Pandey et al. 1997).
Management
Control measures are needed in commercial guava production. There is limited information on resistant or tolerant varieties (Misra 2004; Rahman et al. 2013). Cultural control practices include: pruning, which increases the amount of light and air movement and therefore reduces the time for plant tissues to dry after rainfall; use of micro-irrigation to avoid wetting flowers and fruit; and judicious use of fungicides. Chemical control can be effective, and several systemic and non-systemic fungicides are available for use on guava in Florida. Contact your UF/IFAS Extension office for information on registered pesticides. Fungicides should be applied before and during flowering and fruit development; subsequent applications may reduce preharvest and postharvest disease on fruit. Keep pruning tools clean and remove infected plant debris to avoid spore dispersal.
Guava Fruit Scab or Fruit Canker
Symptoms
This disease generally occurs on green fruit and rarely on leaves (Figure 2A–C). The first evidence of infection on fruit is the appearance of minute, brown- or rust-colored, circular necrotic spots. The margins of the spots are raised, giving the appearance of small craters. The canker is confined to a very shallow area and does not penetrate deep into the flesh of the fruit. In severe cases, these cankers may coalesce and tear the skin of the fruit open (Figure 2D). The infected fruits remain underdeveloped, become hard and malformed, and may drop in great numbers (Lim and Manicom 2003).

Credit: Romina Gazis, UF/IFAS TREC
Causal Organism
This disease is caused by Pestalotiopsis psidii and other Pestalotiopsis species (Keith et al. 2006). Pestalotiopsis species are considered weak or opportunistic pathogenic fungi that can colonize the woody stem tissue of twigs asymptomatically (living as an endophyte, inside the plant tissue, causing no symptoms of disease). Pestalotiopsis is easy to identify in artificial nutrient media because it develops white fluffy mycelia with dark fruiting bodies (acervuli), which exude abundant spores (conidia) into shiny black liquid matrix droplets. Conidia are typically 5-celled, brown in color with possible variation in pigmentation intensity, oblong, and clavate to elliptical with three apical appendages; the basal cell is obtuse, with a small pedicel (Figure 2E).
Disease Cycle and Epidemiology
Ideal conditions for this disease occur at 25°C–30°C and at high relative humidity. This pathogen can remain asymptomatic inside woody tissue (twigs or stems) and become pathogenic when the host is under stress. Environmental conditions producing stress are drought, waterlogging, wind damage, transplant shock, mechanical damage caused by pruning, and damage by other pathogens and insects. The fungus invades and colonizes the fruit through insect openings or wounds, and later produces abundant spores which can start a new infection cycle. Spores are spread by rain splash.
Management
When needed, application of insecticides to reduce insect feeding and therefore prevent fruit wounding is recommended. Spacing trees to prevent canopy crowding and shading of adjacent trees and pruning the canopy to increase light and air movement could help to reduce fruit disease incidence (Lim and Manicom 2003). Potential sources of guava resistance to scab/canker disease have been documented in Hawaii (Keith and Zee 2010).
Stylar End Rot
Symptoms
Symptoms start as a restricted discoloration in the region lying below the persistent calyx (Figure 3A–B). As the fruit ripens, the discolored area rapidly expands, becoming dark brown and soft and appearing "water soaked." Along with the discoloration of the peel, the flesh tissue also becomes discolored and rots (Figure 3C). Advanced disease symptoms include fruit shrinking and the appearance of concentric wrinkles on the fruit’s peel. Finally, the symptomatic tissue is covered with small, dark, fruiting bodies (pycnidia). Losses ranging from 10% to 60% have been reported in orchards in India (Misra 2005; Prakash and Pandey 2005). Disease symptoms can be seen while fruits are still attached to the trees (preharvest) but are most commonly observed during fruit transportation and storage (postharvest) (Misra 2007).

Credit: Romina Gazis, UF/IFAS TREC
Causal Organism
This disease is mainly caused by Phomopsis psidii, although other organisms have been reported to cause similar symptoms, including generalist fruit rot fungi in the Botryosphaeriaceae family (i.e., Botryosphaeria dothidea, Neofusicoccum parvum, and Neofusicoccum ribis). Phomopsis and Botryosphaeriaceae-caused stylar end rot can be differentiated by incubating the symptomatic fruits under high humidity. Botryosphaeriaceae fungi produce a thick, dark mycelium mat on top of the fruit lesions while Phomopsis species produce fruiting bodies (pycnidia) without much mycelial growth (Nogueira Júnior et al. 2016). In Florida, both groups of pathogens have been recovered from symptomatic tissue; however, the role of each organism in inducing disease symptoms has not been investigated.
Disease Cycle and Epidemiology
Wounded fruit are more susceptible to infection and pathogen colonization (Rai 1956). The best conditions for disease development are high temperatures (28°C to 31°C) and prolonged periods of wetness (Nogueira Júnior et al. 2016). Stylar end rot is mostly a quiescent disease; fruit infection occurs in the field and the symptoms develop postharvest. Infected fruit left on the tree could become mummified and serve as inoculum for the following season. There are reports of Phomopsis psidii causing twig blight in guava, suggesting an endophytic stage within the life cycle of the pathogen (Misra 2004).
Management
Removal of infected fruit can help reduce disease pressure. Increasing plant spacing and selectively pruning branches to facilitate aeration and sunlight can reduce the chances of the pathogen germinating and infecting the fruit. The application of copper, Chlorothalonil, and Mancozeb fungicides has been recommended (Plantwise, CABI). Efficacy of labeled fungicides in Florida has not been assessed.
Root Rot Diseases Caused by Fusarium and Phytophthora Species
Root rot diseases are common in areas where the soil has poor drainage and plants (usually young ones) are overwatered. Roots need oxygen. When there is not enough available in the rhizosphere, roots become stressed and start to decay. This process creates wounds in the root tissue that allow the entrance of common soilborne pathogens such as species of Fusarium and Phytophthora. Even though these two pathogenic genera are not related, they have some similarities in their epidemiology. They are both considered common inhabitants of soil and they can spread through the movement of soil and water splash. Phytophthora produces motile zoospores; thus, it can independently move across the orchard through free water. Both pathogens produce abundant propagules so the diseases can spread quickly.
Symptoms
Because these pathogens affect the roots of the host, external symptoms in the canopy can be similar to those of nutrient deficiency, including leaf chlorosis, stunting, and defoliation. When the plant is removed from the soil, necrosis can be observed on root tissue, and the root system will appear underdeveloped (Figure 4A–C).
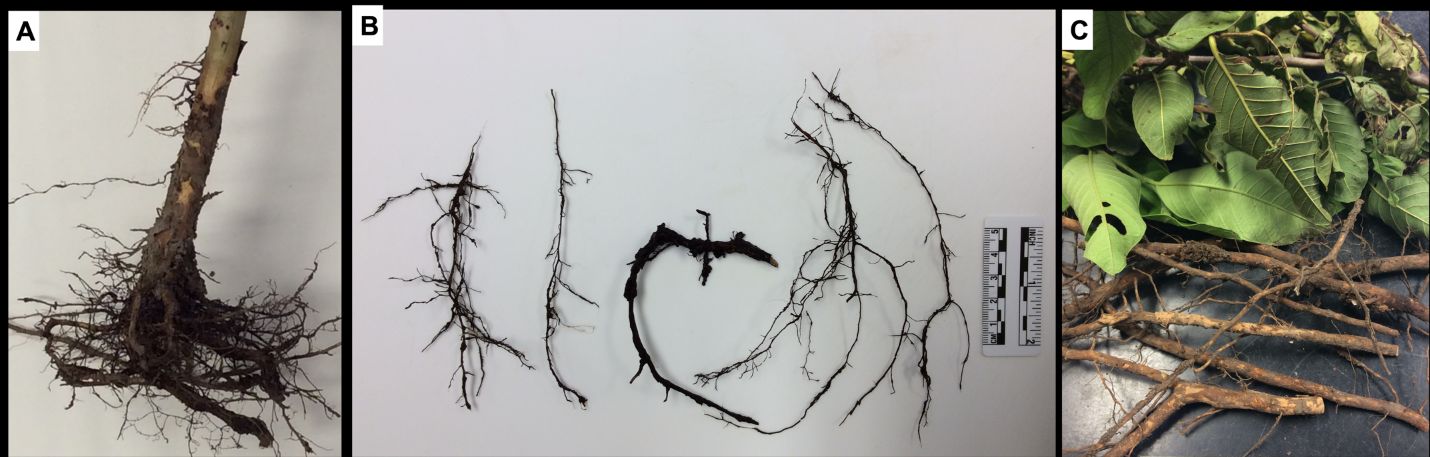
Credit: Romina Gazis, UF/IFAS TREC
Management
Cultural practices are extremely important to avoid root rot problems. Be sure to plant guava in soils with good drainage and provide proper irrigation.
Guava Diseases Caused by Non-Fungal Agents
Algal Leaf Spot
Algal leaf spot occurs on a wide range of tropical fruit species. On most hosts, leaf infection is of little direct economic importance and is confined to low-hanging branches near ground level. However, on guava, algal leaf spot can cause significant tissue necrosis, extensive defoliation, economic injury to fruit quality, crop loss, and reduced photosynthesis and plant vigor (Nelson 2008). Susceptibility to the disease is greatest when suboptimal environmental conditions such as poor soil management (e.g., flooding, excessive irrigation), tree overcrowding, and weed pressure are present. The presence of insects, mites, and other foliar pathogens may further increase the severity of the disease. In Florida, this disease has not been observed to affect the fruit directly.
Symptoms
Symptoms can be seen on both lower and upper leaf surfaces. Leaf spots appear initially as tiny, dark-brown specks that enlarge into roughly circular lesions with ash-colored centers and dark-brown to blackened margins (Figure 5A–D). In some cultivars, spots are surrounded by a yellow halo. As the disease progresses, orange, rust-colored, dense silky tufts appear in the center of the leaf spots. Upon scraping away these spots, a thin, grayish-white to dark-colored dead crust remains on the leaf. These spots usually come together to form large irregular patches on a leaf. Algal leaf spot may result in leaf drop. Twigs and branches are also affected, causing the bark to crack due to the growth and expansion of the pathogen’s filaments into the cortical tissues of the host.
Causal Organism
Two species of parasitic green algae, Cephaleuros virescens and C. parasiticus, are the causal pathogens of this disease. The orange color of spots is the thallus (body) of the alga (Figure 5C–E). The structure of the thallus consists of cells from which erect, bristle-like branches arise (Figure 5E). The apical parts of some cells swell to form enlarged support cells that produce several stalked, terminal, or ovoid sporangia (spore-producing structures). The sporangia of the alga produce zoospores with two hairlike structures (flagella) that allow them to swim. These zoospores are released when free water is present. Once released, they fuse into pairs, giving rise to the next generation of algae.
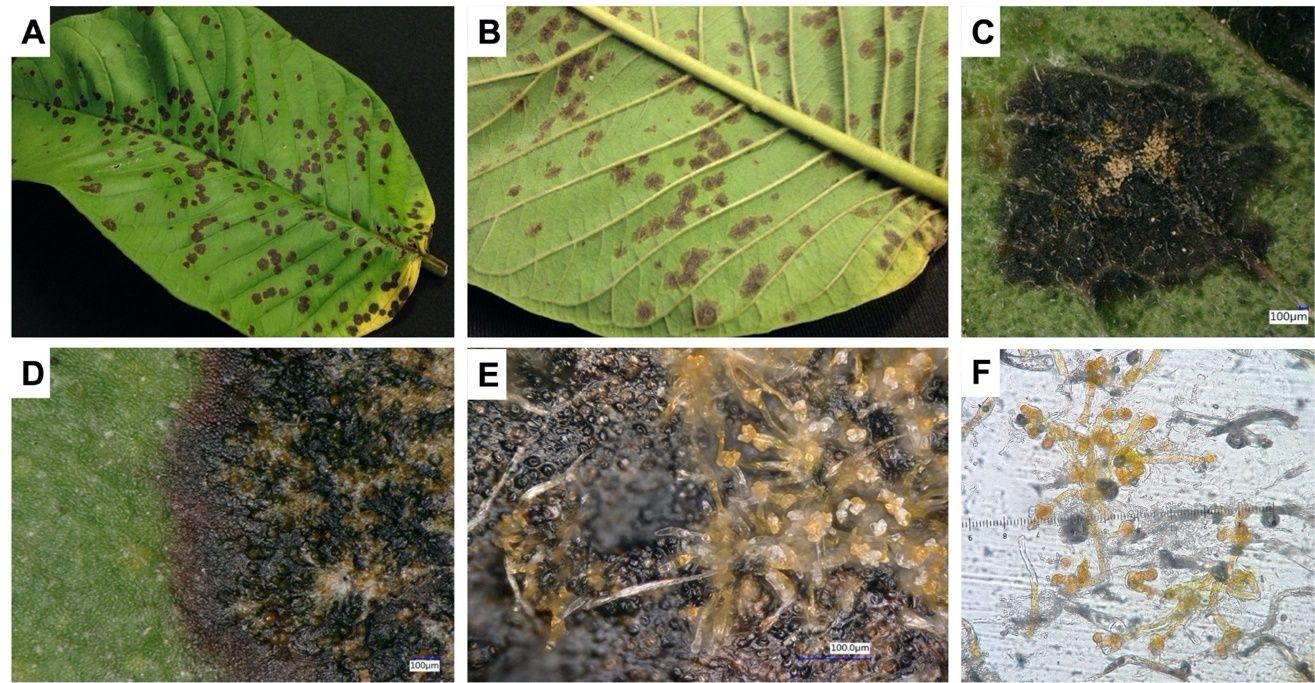
Credit: Georgina Sanahuja, former member of the UF/IFAS TREC PDC (Sanahuja et al. 2018)
Disease Cycle and Epidemiology
Algal leaf spots proliferate under wet or humid conditions within the trees’ canopy. Infectious propagules are disseminated by water splash and wind; biflagellate zoospores (spores that have two flagella) are the primary infection stage of the pathogen.
Management
Algal leaf spot can be reduced by maintaining tree vigor with cultural techniques such as proper fertilization and irrigation, proper pruning to enhance air circulation within the canopy and sunlight penetration, managing weeds, and using wider tree spacing. Managing insects, mites, and other foliar diseases increases tree vigor and lessens susceptibility to this disease. If chemical control is warranted, periodic applications of a copper-based fungicide will control the algae.
Nematodes
Sixteen nematode genera have been reported in guava (Lim and Manicom 2003). Depending on soil type, different nematode species can proliferate and cause disease. A general decline in tree vigor is observed in response to high nematode populations.
Symptoms
Aboveground symptoms associated with nematode infection include chlorosis, stunting, stem and branch dieback, premature wilting, reduced leaf size and yield, and nutrient deficiencies (Figure 6A). Below the ground, a reduction of fine root densities and root distortion are observed (Figure 6B).
Causal Organism
Causal nematode agents include reniform nematode (Rotylenchulus reniformis), burrowing nematode (Radopholus similis), ring nematode (Hemicriconemoides magiferae), and root-knot nematode (Meloidogyne spp.). While many species of root-knot nematode (M. incognita, M. arenaria, M. javanica, and M. hapla) cause disease in guava-producing areas worldwide, Meloidogyne incognita, races 1 and 2 are pests of guava in Florida (Lim and Manicom 2003).

Credit: Romina Gazis, UF/IFAS TREC
Management
Exclusion is the best control method. This can be achieved at the nursery stage by using non-infested plant material and disinfested (clean) soil or artificial media (Lim and Manicom 2003). In Florida, there are no registered nematicides for use on guava. Once nematodes are in the orchard, it is very difficult to eradicate them. Addition of compost to the soil around the trees (kept 0.5 to 1 ft from the trunk) can provide a soil environment that suppresses nematode populations and enhances guava root growth.
Other Diseases Reported in Florida But Not Commonly Seen
Guava Rust
Rust diseases can be very destructive to guava-producing regions around the world. The pathogen has been reported in Central and South America, the Caribbean, and Florida. Aside from guava, the disease affects other members of the Myrtaceae family including allspice (Pimenta dioica), eucalyptus (Eucalyptus spp.), and melaleuca (Melaleuca quinquenervia) (Alfieri et al. 1994). In Florida, the disease has not been reported on guava but on other hosts such as Simpson’s stopper (Myrcianthes fragrans), myrtle (Myrtus communis), bottlebrush (Callistemon viminalis), rose apple (Syzygium jambos), brush cherry (S. paniculatum), java plum (S. camini) (Loope and La Rosa 2010), and on the genus Heteropyxis (Alfenas et al. 2005). Even though the disease has not been observed affecting guava production, Rayachhetry et al. (2001) showed that the guava rust strains present in Florida are able to induce disease in Florida-grown guava trees.
Symptoms
The pathogen can affect foliage, young shoots, inflorescences, and fruit of guava. Typical symptoms associated with this disease include tissue distortion, defoliation, reduced growth, stem dieback, and, if severe, mortality. Conspicuous orange to reddish pustules of the causal fungus are produced on foliage, young shoots, flowers, and fruit (Lim and Manicom 2003).
Causal Organism
The rust pathogen, Austropuccinia psidii (formerly Puccinia psidii), is an obligate parasite in the order Uredinales (Basidiomycota). It produces pale-yellow, relatively large spores (0.1–0.5 mm in diameter) beneath the leaf epidermis, later becoming erumpent in groups on brownish or blackish spots (Lim and Manicom 2003). This fungus produces two kinds of spores: urediniospores (during the summer) and teliospores (during the winter); however, teliospores have not been observed in Florida (Burnett and Schubert 1985). Urediniospores are globose, ellipsoid to obovoid, with finely echinulate cell walls. If observed, teliospores are ellipsoid to oblong, rounded above and narrow below, slightly constricted at the septum, pale yellow in color, and smooth.
Disease Cycle and Epidemiology
Infection of upper and lower leaves occurs in wet to moist environments within a temperature range of 13°C–25°C (Burnett and Schubert 1985). Urediniospores germinate at temperatures in the range of 18°C–22°C. Disease usually begins at the onset and development of young shoots; leaves that are 40 days or older have been observed to be more resistant to infection. Dissemination occurs via rain splash (Holliday 1980).
Management
Fungicides are needed to control guava rust. Scouting fields for onset of disease or during the times of year when environmental conditions are favorable for pathogen infection are recommended, so that proper and timely fungicide applications can be made. In addition, proper cultural tactics such as pruning to open the tree canopy to light and air movement, proper fertilization, irrigation, and sanitation aid in achieving a healthy, vigorously growing tree less vulnerable to disease pressure.
Mushroom Root Rot
Mushroom root rot is a common and widespread disease that affects conifers and hardwoods in Florida. Aside from guava, the disease has been reported on over 200 species of trees and shrubs (Ash and Barnard 1994).
Symptoms
Infected trees will usually not show any symptoms until the disease has weakened a significant portion of the root system. Diseased trees may exhibit a variety of symptoms, including thinning of the crown, yellowing of foliage, premature defoliation, branch dieback, decaying roots, and lesions at the root collar. On some occasions, a tree may show symptoms of decline for several years before dying, whereas in other instances, trees may die rapidly without any visible symptoms.
Causal Organism
Mushroom root rot, caused by Desarmillaria tabescens (formerly Armillaria tabescens), can cause severe problems in guava plantings. The most notable sign of disease is the characteristic fruiting bodies (mushrooms/sporocarps) which develop near the base of infected trees, although sporocarp production is not common in Florida. The mushrooms usually appear in large clusters at the bases of trees during fall, but fruiting can occur at other times. The mushrooms, when fresh and young, are covered with dark-brown scales; at maturity, the scales are often concentrated near the center and vaguely radially arranged, tan to tawny brown or cinnamon brown, sometimes yellow to yellowish, with gills beneath the cap running down the stem, and lacking an annulus around the stem (Sanagorski et al. 2013). Spores are ellipsoid and smooth and have a prominent short, blunt, off-center spike (called an apiculus or hilar appendage). Production of mushrooms is not always guaranteed, even if the tree is heavily infected by the fungus. When a tree is in decline, and the mushrooms are absent, the fungus can be identified by the characteristic cream-colored mycelium just beneath the bark of infected roots and tree bases (Sanagorski et al. 2013).
Disease Cycle and Epidemiology
This fungus tends to spread vegetatively, via threadlike fungal strands (hyphae and mycelium) from infected roots to healthy roots through root-to-root contact. Initial infections by the pathogen are presumed to be via airborne basidiospores released from the gills on the undersides of the mushrooms. The removal of infected plants does not eradicate the pathogen, which survives as a saprobe in stumps and dead roots and may cause infections for several years. Infected plants may not show any visible symptoms until a major portion of the root system is affected. The fungus can remain on the root system and feed without obvious injury to the plant. If plants are grown under optimum conditions with no stress, the plant can withstand the damage to the roots caused by the pathogen by continuously producing new, uninfected roots. However, if environmental conditions are not optimal and the plant is stressed, the pathogen can cause extensive damage leading to the rapid decline and eventual death of the plant without exhibiting obvious symptoms. In some instances where the environment is optimal for the fungus, rapid colonization and spread onto new and existing roots will cause a quick decline of a plant even though growing conditions are optimal for plant growth and development.
Management
Preventative measures to manage mushroom root rot such as the removal of diseased trees and their root system before replanting should be taken to reduce the possibility of future infections. Cultural practices to maintain vigorously growing plants help to reduce the colonization of the fungus. These practices include keeping root damage to a minimum when planting or transplanting, planting at proper depths so that the root collar is not buried in the soil, keeping mulch away from the root collar, and maintaining an optimum growing environment (i.e., soil fertility, pH, irrigation) to alleviate any stresses to the plant. Addition of organic matter such as compost and/or mulch to the soil may reduce the severity of mushroom root rot by promoting pathogen-antagonistic organisms and enhancing the soil environment for additional root growth. Currently, there are no known effective fungicides registered for the control of mushroom root rot on guava in Florida (Ploetz et al. 2003).
References
Alfenas, A. C., E. A. V. Zauza, M. J. Wingfield, J. Roux, and C. M. Glen. 2005. “Heteropyxis natalensis, a New Host of Puccinia psidii Rust.” Australasian Plant Pathology 34 (2): 285–286. https://doi.org/10.1071/AP05023
Alfieri, S., J. Langdon, K. R. Kimbrough, N. E. El-Gholl, and C. Wehlburg. 1994. “Diseases and Disorders of Plants in Florida (Bulletin No. 14).” Florida Department of Agriculture and Consumer Services. Contribution 680:1114.
Ash III, E. C., and E. L. Barnard. 1994. “Mushroom Root Rot.” In Forest and Shade Tree Pests. Division of Forestry. Leaflet 11.
Burnett, H. C., and T. S. Schubert. 1985. “Puccinia psidii on Allspice and Related Plants.” Florida Department of Agriculture and Consumer Services.
Center for Agriculture and Bioscience International (CABI), Plantwise Knowledge Bank (PWKB). Accessed December 4, 2023. https://plantwiseplusknowledgebank.org/
Holliday, P. 1980. Fungus Diseases of Tropical Crops. 147. Cambridge: University Press.
Hossain, M. S., and M. B. Meah. 1992. “Prevalence and Control of Guava Fruit Anthracnose.” International Journal of Pest Management 38 (2): 181–185. https://doi.org/10.1080/09670879209371680
Keith, L. M., M. E. Velasquez, and F. T. Zee. 2006. “Identification and Characterization of Pestalotiopsis spp. Causing Scab Disease of Guava, Psidium guajava, in Hawaii.” Plant Disease 90 (1): 16–23. https://doi.org/10.1094/PD-90-0016
Keith, L. M., and F. T. Zee. 2010. “Guava Diseases in Hawaii and the Characterization of Pestalotiopsis spp. Affecting Guava.” II International Symposium on Guava and Other Myrtaceae 849:269–276. https://doi.org/10.17660/ActaHortic.2010.849.31
Lim, T. K., and B. Q. Manicom. 2003. “Diseases of Guava.” Diseases of Tropical Fruit Crops:275–289. https://doi.org/10.1079/9780851993904.0275
Loope, L., and A. M. La Rosa. 2010. “An Analysis of the Risk of Introduction of Additional Strains of the Rust Puccinia psidii Winter ('Ohi'a Rust) to Hawai'i (No. 2008-1008).” US Geological Survey. https://doi.org/10.3133/ofr20081008
Misra, A. K. 2004. “Guava Diseases—Their Symptoms, Causes and Management.” In Diseases of Fruits and Vegetables: Volume II: Diagnosis and Management. 81–119. Dordrecht: Springer Netherlands. https://doi.org/10.1007/1-4020-2607-2_4
Misra, A. K. 2005. “Present Status of Important Diseases of Guava in India with Special Reference to Wilt.” I International Guava Symposium 735:507–523. https://doi.org/10.17660/ActaHortic.2007.735.68
Misra, A. K. 2007. “Post-Harvest Diseases of Guava and Their Management.” In Biotechnology: Plant Health Management, edited by Neeta Sharma and H. B. Singh. 397–419.
Nelson, S. C. 2008. “Cephaleuros Species, the Plant-Parasitic Green Algae.” University of Hawaii at Manoa, College of Tropical Agriculture and Human Resources, Cooperative Extension Service.
Nogueira Júnior, A. F., I. H. Fischer, C. A. Bragança, N. S. Massola Junior, and L. Amorim. 2016. “Identification of Botryosphaeriaceae Species That Cause Stylar-End Rot of Guavas and Characterization of the Disease Monocycle.” European Journal of Plant Pathology 144:271–287. https://doi.org/10.1007/s10658-015-0765-x
Pandey, R. R., D. K. Arora, and R. C. Dubey. 1997. “Effect of Environmental Conditions and Inoculum Density on Infection of Guava Fruits by Colletotrichum gloeosporioides.” Mycopathologia 137 (3): 165–172. https://doi.org/10.1023/A:1006842801828
Ploetz, R. C., T. K. Lim, J. A. Menge, K. G. Rohrbach, and T. J. Michailides. 2003. “Common Pathogens of Tropical Fruit Crops.” In Diseases of Tropical Fruit Crops. 1–19. Wallingford, UK: CABI Publishing. https://doi.org/10.1079/9780851993904.0001
Prakash, O. M., and B. K. Pandey. 2005. “Current Scenario of Guava Diseases in India and Their Integrated Management.” I International Guava Symposium 735:495–505. https://doi.org/10.17660/ActaHortic.2007.735.67
Rahman, M. A., T. H. Ansari, M. B. Meah, and T. Yoshida. 2003. “Prevalence and Pathogenicity of Guava Anthracnose with Special Emphasis on Varietal Reaction.” Pakistan Journal of Biological Sciences. https://doi.org/10.3923/pjbs.2003.234.241
Rai, J. N. 1956. “Stylar End Rot of the Guava Fruit (Psidium guayava Linn).” Proceedings/Indian Academy of Sciences 43 (1): 55–61. New Delhi: Springer India. https://doi.org/10.1007/BF03050219
Rayachhetry, M. B., T. K. Van, T. D. Center, and M. L. Elliott. 2001. “Host Range of Puccinia psidii, a Potential Biological Control Agent of Melaleuca quinquenervia in Florida.” Biological Control 22 (1): 38–45. https://doi.org/10.1006/bcon.2001.0949
Sanagorski, L., A. Trulock, and J. A. Smith. 2013. “Armillaria Root Rot (Also Known as Mushroom Root Rot, Shoestring Root Rot, Honey Mushroom Rot): ENH1217/EP478, 7/2013.” EDIS 2013 (8). https://doi.org/10.32473/edis-ep478-2013
Sanahuja, G., P. Lopez, A. J. Palmateer, and A. R. Chase. 2018. “Red Rust of Neoregelia Bromeliads Caused by a Parasitic Alga Cephaleuros parasiticus in Florida.” Plant Health Progress 19 (1): 27–33. https://doi.org/10.1094/PHP-11-17-0068-RS