Information contained in this publication is intended for Florida blueberry growers to use as a guide in the identification and management of anthracnose on southern highbush blueberries (SHB). For more information, search the Ask IFAS website (https://edis.ifas.ufl.edu) or contact your local UF/IFAS Extension office (https://sfyl.ifas.ufl.edu/find-your-local-office/).
Anthracnose Diseases and Symptoms
Anthracnose is a general name given to diseases caused by a group of fungal pathogens in the genus Colletotrichum. These pathogens affect a wide range of plants, including fruit crops, trees, turfgrass, and vegetable crops. Anthracnose diseases occur in both northern and southern US blueberry production regions, but are most problematic in tropical and subtropical regions of the eastern United States (Cannon et al. 2012). Symptoms of anthracnose on blueberry occur on leaves, twigs, canes, blossoms, and fruit.
Fruit Symptoms
Anthracnose fruit rot is also referred to as "ripe rot." Infection may occur as early as bloom, with symptoms appearing as the fruit begins to ripen, including shriveling, development of sunken lesions, soft, rotted fruit, and eruption of orange- or salmon-colored spore masses on the blossom end of the fruit (Figure 1). In some cases, symptoms do not appear until after the fruit is harvested and stored (Polashock et al. 2017). Fungicide applications from bloom through harvest prevent significant ripe rot losses most years when coupled with frequent hand-harvesting and rapid-cooling practices that are standard for SHB growers in Florida. Pre-harvest fungicides are especially important in years where there is a high incidence of disease in the field coupled with warm, wet weather, which can promote disease development.

Credit: M. Velez-Climent
Leaf Symptoms
Leaf spot symptoms of anthracnose are common after harvest in Florida, and the severity differs by variety, with 'Jewel' considered to be one of the most susceptible. Symptoms occur mostly at the edge of the leaves and include circular or irregular-shaped, dime- to nickel-sized, brown- to dark-brown spots with distinct concentric circles of discoloration (bulls-eye patterns). Small, black fungal fruiting bodies called acervuli appear as the lesions mature, as well as orange spore masses under humid conditions (Figure 2). Postharvest fungicides are used to prevent defoliation through flower bud initiation in the fall (Harmon 2014).

Credit: M. Velez-Climent and P. Harmon
Stem Symptoms
Stem symptoms typically do not warrant management for most commercial varieties. However, severe dieback and plant losses do occur on the varieties 'Flicker' and 'Scintilla' in central Florida when resistance to the QoI (strobilurin) class of fungicides (e.g., Abound, Cabrio, Pristine) occurs in the pathogen population. Stem symptoms include dark, sunken lesions on young, succulent canes (Figure 3), which can become brittle and break where lesions progress, resulting in a general decline of the bush. Dieback of branch tips, twigs, and fruit spurs can also be observed following anthracnose infection (Figure 4). Orange sporulation (Figure 5) can be observed erupting from blister-like acervuli in the lesions. Stem symptoms are most severe during hot, humid summer months, continuing through the fall, whenever conditions are favorable. Other diseases of SHB, such as stem blight or Phomopsis twig blight, can cause symptoms similar to those of anthracnose during the same time of year. When in doubt, submit a plant disease sample to the UF/IFAS Plant Diagnostic Center for confirmation (http://plantpath.ifas.ufl.edu/extension/plant-diagnostic-center/).

Credit: P. Harmon

Credit: P. Harmon

Credit: P. Harmon
Blueberry Variety Resistance
'Flicker' is the most susceptible variety to stem infection, followed by 'Scintilla.' Dieback due to anthracnose disease has resulted in several growers removing 'Flicker' plants from production in central Florida, and 'Flicker' is no longer recommended for new plantings in Florida. Commercial varieties including 'Chickadee', 'Emerald', 'Farthing', 'Jewel', 'Kestrel', 'Rebel', 'San Joaquin', 'Springhigh', and 'Star' have been tested in UF research and are not susceptible to anthracnose stem infections. Additional testing is underway to characterize susceptibility in additional cultivars.
Anthracnose Pathogens and Disease Cycle
UF research has shown that anthracnose pathogens from SHB are mostly in the C. gloeosporioides species group (Figure 6) and some in the C. acutatum group. Isolates from stems can infect fruit and leaves, and vice versa. When fungicide resistance occurs, fungicides with QoI active ingredients are no longer effective tools for preventing ripe rot, defoliation, or the anthracnose dieback disease on the 'Flicker' variety.
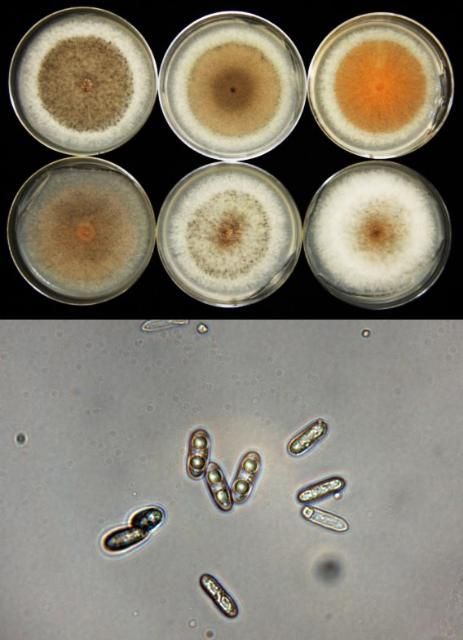
Credit: M. Velez-Climent and P. Harmon
The pathogen overwinters in infected twigs, canes, old fruit spurs, fruit, and bud scales, sporulating in the spring during periods of prolonged warm, wet weather. Spores are produced in a sticky mass that can be spread to new tissues by splashing water, either by rain or overhead irrigation (which has been correlated with more severe outbreaks compared to drip irrigation). Sticky spores can also be spread by workers and equipment during pruning and harvest. Latent infections on green fruit may not be visible until fruit ripens and spores serve as an inoculum source for the summer.
Disease Management
Anthracnose fruit rot is primarily controlled by fungicides. Azoxystrobin (Abound), Cyprodinil + fludioxonil (Switch), and Pyraclostrobin + boscalid (Pristine) have all proven to be effective in most cases. However, resistance to azoxystrobin is common in central Florida. Inclusion of a captan product in a tank mix is a recommendation where resistance is present. No more than two sequential applications of QoI fungicides are to be made before changing to a fungicide with a different mode of action.
The same fungicides are typically effective for the control of anthracnose leaf spot and should be applied immediately after harvest. Other fungicides that are generally effective include mono- and di-potassium salts of phosphorous acid (AgriFos,K-Phite) and potassium phosphite (ProPhyt).
Historically, QoI fungicides have been effective for the control of anthracnose stem lesions. However, growers in central Florida have experienced limited success in recent years, due in part to the development of fungicide resistance. In particular, QoI fungicides such as Abound, Cabrio, and Pristine have proven to be ineffective in controlling this form of anthracnose where resistance is present. In that situation, recommendations for control include rotations of DMI fungicides (including Tilt and Orbit, Indar, Quash, and Proline) with compatible contact fungicides (such as Bravo, Captan, Kocide, Omega, and Ziram). Application of fungicides should begin after harvest and continue as long as warm, wet weather conditions remain. In order to provide optimal disease control, calibrate spray equipment for fungicide delivery in sufficient water volume and pressure, to attain sufficient cane coverage and canopy penetration. See 2017 Florida Blueberry Integrated Pest Management Guide for application frequency recommendations for specific fungicides―https://edis.ifas.ufl.edu/hs380.
Pruning out infected stems can help to reduce the amount of the pathogen that is present in the fields. However, this should be done when the foliage is dry, and pruning equipment should be sanitized with bleach or alcohol as frequently as practical during the process. Removing and destroying dead plants also helps to reduce the accumulation of inoculum.
On farms where resistance is already present, growers may consider replanting with varieties other than 'Flicker' if required management inputs become cost prohibitive. Using healthy, disease-free plants for new plantings is also important.
For those farms not experiencing resistance, it is important to avoid the overuse of QoI fungicides to minimize selection for resistance. Label recommendations for the maximum seasonal use rate of fungicides with the same active ingredient should be followed. Rotating between fungicides in different FRAC groups can reduce the probability of resistance. Newer fungicides that are prone to disease resistance development can also be mixed with older ones that are not. If you suspect resistance, send a sample to the UF/IFAS Plant Disease Clinic and ask for disease resistance testing.
For additional information concerning fungicide products and other integrated approaches to blueberry production in Florida, see the UF/IFAS Blueberry Integrated Pest Management Guide (https://edis.ifas.ufl.edu/hs1156).
References
Cannon, P. F., U. Damm, P.R. Johnston, and B.S. Weir. 2012. "Colletotrichum-current status and future directions." Stud. Mycol. 73: 181–213.
Harmon, P. F. 2014. "The Blueberry News." Vol 3, Issue 2, Winter 2014: 34, 36. Print.
Polashock, J., F. Caruso, A. Averill, and A. Schilder, eds. 2017. "Infectious Diseases." In Compendium of Blueberry, Cranberry, and Lingonberry Diseases and Pests. 10–12. St. Paul, MN: The American Phytopathological Society.