The Purpose and Audience
Florida pasture grasses harbor fungal endophytes that produce secondary metabolites. Some of these metabolites may be detrimental to the livestock that ingest them. Mycotoxins present in Florida pastures raised the concerns of area ranchers as a possible contributing, negative factor impacting livestock health. Florida Cattlemen’s Association noted these concerns and funded our team to investigate the distribution of mycotoxins in Florida pastures. This article reports mycotoxins identified from Florida’s dominant pasture grass species, bahiagrass. The target audience of this article includes livestock producers, Extension professionals, and others interested in mycotoxins originating from bahiagrass and smutgrass. The information will evoke awareness by Extension professionals and producers regarding the state of mycotoxin assessment in Florida bahiagrass pastures, which can be used by producers in consultation with their veterinarian about their own livestock health concerns.
Introduction
All forage crops harbor fungi, and among them, some are helpful by aiding with forage growth and nutrition through helping to obtain plant essential nutrients from the soil and atmosphere. Under certain circumstances the fungi living in forage tissue may produce “secondary metabolites.” Some fungal species produce secondary metabolites called mycotoxins, which are toxic when ingested by animals— in this case, cattle. Indeed, in recent years, beef cattle ranchers in Florida reported some health issues in cattle grazing on bahiagrass and bermudagrass pastures. The incidents were not attributed to animal nutritional imbalances or other obvious causes. As targets were eliminated, attention shifted to potential causes related to forage consumption.
The Fungal Produced Mycotoxins Identified in Bahiagrass Pastures
What are the roles of endophytes, epiphytes and mycotoxins in pasture systems? “Endophytes” and “epiphytes” are groups of microorganisms (including fungi) living in and on plant tissues, respectively, without causing any symptoms. For example, leaf wilting, leaf spots, or other visible features are symptoms of plant leaf pathogens, thus indicating a condition of disease, not a presence of endophytes/epiphytes. Although the endophytes/epiphytes do not seem to impact forage yield, they may produce secondary metabolites. In this instance, the secondary metabolites produced by endophytes/epiphytes are not essential for their development and reproduction but may help these microbes be more competitive in the environment.
In comparison, some secondary metabolites are considered potential mycotoxins, but the prevalence, dose, and interactions with other mycotoxins are not well studied. In 2017, a team of state specialists and Extension agents sampled bahiagrass pastures and an often-associated pasture weed, smutgrass, from four Florida counties (Gadsden, Osceola, Hillsborough, and Hardee) (Figure 1). The goal was to identify the co-occurrence of mycotoxins and the mycotoxin producers (the fungi) using liquid chromatography, mass spectrometry, and DNA analysis. We found that the leaves of bahiagrass and smutgrass harbor different dominant fungal groups. For example, in this investigation, the dominant leaf fungal genera identified from bahiagrass were Ramichloridium, Dissoconium, and Curvularia; and from smutgrass they were Papiliotrema, and Preussia. The identification of these fungi, together with other dominant fungal groups, is necessary in helping determine potential mycotoxin occurrence and concentrations in ranchers’ pastures. The dominant mycotoxins identified from site-collected bahiagrass samples included alternariol methyl ether, beauvericin, beta-zearalenol, dihydrolysergol, emodin, and fumonisin B1. It is interesting to note that Alternaria and Fusarium were not the most dominant but still the common fungal genera identified in bahiagrass leaves, having ranked in the top twenty of identified fungal taxa. However, the predominant toxins identified in this report were mostly produced by Alternaria and Fusarium (i.e., Alternariol methyl ether by Alternaria; Zearalenone, beta-zearalenol, and fumonisin B1 by Fusarium).
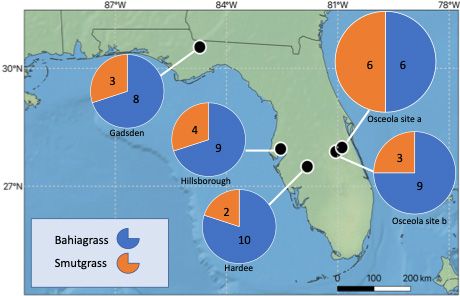
Credit: Hui-Ling Liao and Ko-Hsuan Chen
Of these dominant toxins, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) only evaluated fumonisin B1 and zearalenone for tolerable intake by mammals. Secondary metabolite concentrations varied among our collected grass samples. A small portion of our samples contained higher concentrations of fumonisin B1 than the recommended tolerable intake (Table 1). Currently no data is available for JECFA to evaluate the threshold of mycotoxins in grasses contributing to livestock illness.
Table 1. Percentage of the samples (bahiagrass) that harbor mycotoxins above the tolerable intake level established by the Joint FAO/WHO Expert Committee on Food Additives (JECFA).
Symptomatic Plant Fungi on Bahiagrass
What about the fungi that cause some “symptoms” on bahiagrass leaves? During the 2017 sampling year across four of the five locations (Figure 2), “black stroma fungal structures” were identified (Figure 2B-D). Similar symptoms were observed in both bahiagrass and smutgrass. Through molecular analysis, we identified the black stroma present on bahiagrass leaves were Myriogenospora atramentosa, and the black stroma present on smut grass leaves were Balansia epichloë. The bahiagrass seed heads were occasionally found covered with M. atramentosa tissue (Figure 2E- H). We had to address another question: does fungal species that present “black stroma structure” on leaves also produce secondary metabolites? This required further lab analysis. To date, we know that in the case of bahiagrass, symptomatic leaves were associated with greater concentrations of dihydrolysergol and emodin. Dihydrolysergol is an ergot alkaloid precursor, and it was detected in 30% of symptomatic bahiagrass samples. Ergot alkaloids have been reported to be produced by certain fungal groups (e.g., Claviceps) that affect many types of crops (e.g., rye, wheat, barley, corn, grass and others) and can cause ergotism (ergot poisoning) in animals (Panaccione 2005; Coufal-Majewski et al 2016; Weaver et al 2021; Guerre 2015). Ergot can affect the digestive, cardiovascular, and nervous systems of animals. The symptoms of ergotism may include burning sensations of the arms and legs, seizures, convulsions, paresthesia, muscle pain, diarrhea, abdominal pain, nausea, vomiting, coma, or even death. The dihydrolysergol concentrations were not significantly different between symptomatic and non-symptomatic leaves of bahiagrass (Chen et al. 2022), which suggests that the black stroma (M. atramentosa) was not the main source of dihydrolysergol (Figure 3). Accumulation of emodin showed considerable consistency among grass samples with Myriogenospora atramentosa (Chen et al. 2022). Since emodin can be produced by both fungi and plants, it is possible that the fungi (M. atramentosa) produced emodin or also triggered bahiagrass to produce emodin. Dong et al. (2016) found emodin to be hepatotoxic (damaging to the liver) and nephrotoxic (damaging to the kidneys) in mammals (e.g., rat), leading to reproductive toxicity with high doses and chronic exposure. However, further work is required to determine if emodin intake at concentrations found in Florida bahiagrass impact livestock health.
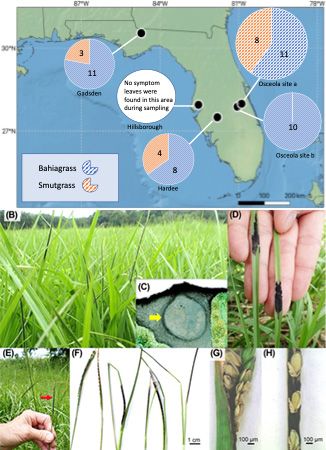
Credit: Map adapted from (Chen et al. 2022) and Figures 2B-H from (Chen et al. 2022)
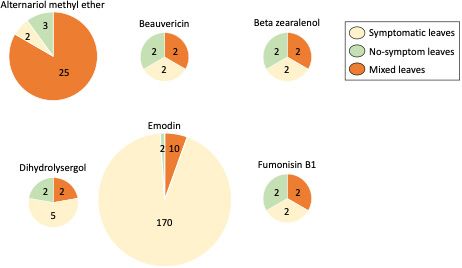
Credit: Adapted from (Chen et al. 2022)
Future research might expand sampling efforts to more locations and across time to further characterize potential seasonal influences on mycotoxin prevalence in Florida pastures. Detectable quantities of mycotoxins and the fungi producing them, both of which are identified by this and other ongoing surveys, indicate a potentially urgent need to develop a rapid and affordable diagnostic station for mycotoxins to better serve livestock Extension specialists and their producer clients.
References
Chen, K-H., F. Marcon, J. Duringer, A. Blount, C. Mackowiak, H-L. Liao. 2022. “Leaf Mycobiome and Mycotoxin Profile of Warm-Season Grasses Structured by Plant Species, Geography and Apparent Black-Stromata Fungal Structure.” Applied and Environmental Microbiology 88(21). https://doi.org/10.1128/aem.00942-22
Coufal-Majewski, S., K. Stanford, T. McAllister, B. Blakley, J. McKinnon, AV. Chaves, Y. Wang. 2016. “Impacts of Cereal Ergot in Food Animal Production.” Frontiers in Veterinary Science 3. https://doi.org/10.3389/fvets.2016.00015
Dong, X., J. Fu, X. Yin, S. Cao, X. Li, L. Lin, Ni J. Huyiligeqi. 2016. “Emodin: A Review of Its Pharmacology, Toxicity and Pharmacokinetics.” Phytotherapy Research 30(8):1207–1218. https://doi.org/10.1002/ptr.5631
Guerre, P. 2015. “Ergot Alkaloids Produced by Endophytic Fungi of the Genus Epichloe.” Toxins 7(3):773-790. https://doi.org/10.3390/toxins7030773
Panaccione, D. G. 2005. “Origins and Significance of Ergot Alkaloid Diversity in Fungi.” FEMS Microbiology Letters 251(1):9-17. https://doi.org/10.1016/j.femsle.2005.07.039
Weaver, A., D. M. Weaver, N. Adams, A. Yiannikouris. 2021. “Co-occurrence of 35 Mycotoxins: A Seven-Year Survey of Corn Grain and Corn Silage in the United States.” Toxins 13(8):516. https://doi.org/10.3390/toxins13080516