Having healthy animals is the wish of every farmer, but keeping animals healthy requires a good herd health management plan. All animals have various defense mechanisms to prevent or deal with infections. Age, nutrition, or management practices can directly affect these defense mechanisms. Additionally, stress due to heat, weaning, malnutrition, infection, transport, and other factors can impact how the immune system reacts to a pathogen attack.
A critical component of any herd health plan is a vaccination protocol. There are many vaccine options available for common livestock; however, few vaccines are explicitly developed for small ruminants or exotic livestock such as deer. While vaccines designed for cattle, horses, sheep, and goats are frequently employed, the efficacy of these vaccines in specialty livestock like deer is scarcely studied. Anecdotal data is the only information available for most livestock vaccines.
How do vaccines work?
Most vaccines induce protection by priming the system to mount an antibody response. When an animal has not been exposed to a specific pathogen or comes in contact for the first time, it can be slow to develop antibodies. Most vaccines work by introducing the body’s system to pathogen-specific proteins. Often, without this “sneak preview” from the vaccine, the animal cannot generate an immune response quickly enough to clear or destroy the pathogen. However, if the animal has a robust immune system or has been vaccinated, it will suppress the pathogen and, in time, clear or significantly reduce the infection.
When an animal recovers from disease or has been vaccinated (Figure 1), specific cells from the immune system will acquire the ability to remember and recognize the pathogens (virus, bacteria, toxin, or parasite) or parts of the pathogen known as antigens. The next time the immune system recognizes these antigens, it will immediately trigger the production of specific antibodies by specialized cells, which will work to destroy the pathogen. An antibody is a protein component of the immune system that circulates in the blood, recognizes foreign substances such as bacteria and viruses, and neutralizes them.
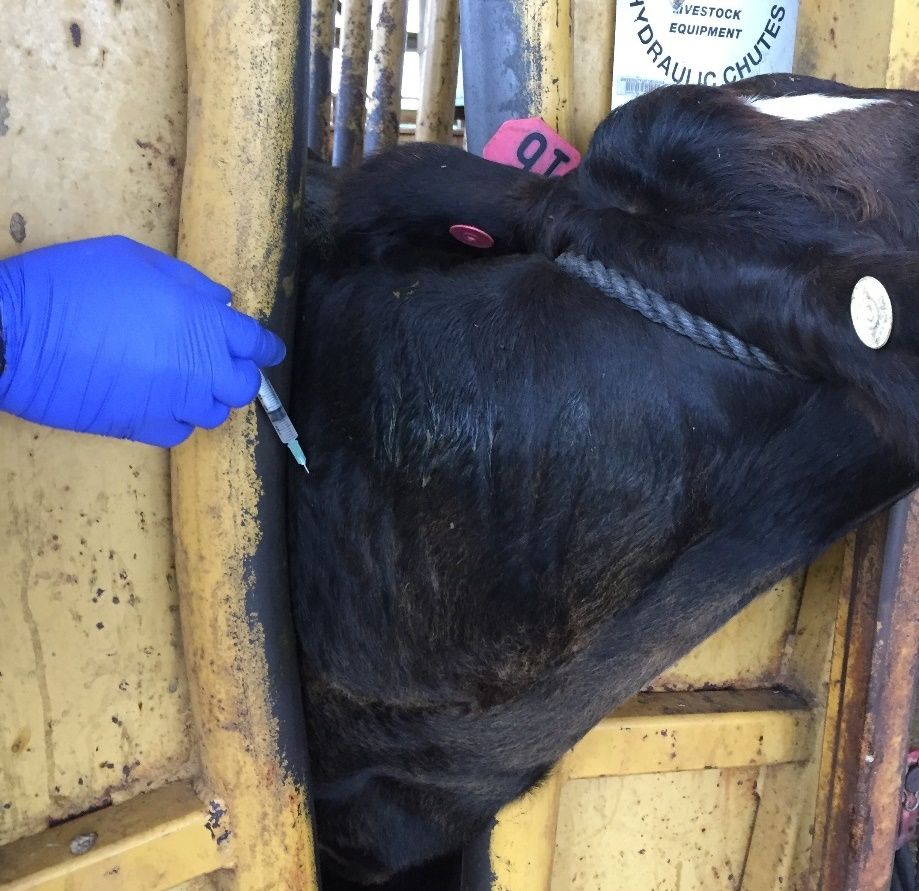
Credit: João Bittar
Vaccines expose the animal to parts of pathogens and challenge the immune system to react to a possible pathogen invasion by creating memory cells for the antigens belonging to that specific pathogen. In the future, if the animal is exposed to the same pathogen, the immune system will quickly generate a response before the pathogen can cause disease. Each antibody is usually specific for only one antigen. Because of this, the immune system keeps a supply of millions of different antibodies on hand to be prepared for any infectious agent. For a naïve animal (an animal that never was exposed to the pathogen), it may take 7 to 14 days after exposure to a pathogen for the body to develop immunity to an antigen. Unfortunately, this is plenty of time for some pathogens to wreak havoc on the body. It often takes only 48 hours for a vaccinated animal to mount an immune response to the same antigen.
There are several types of vaccines used in humans and animals. Most of the licensed veterinary vaccines currently in use are inactivated (i.e., killed) vaccines, live-attenuated vaccines, or toxoids. Each vaccine type uses different strategies to reduce the risk of illness while retaining the ability to induce a beneficial immune response.
Types of Vaccines
- Attenuated vaccine: Some vaccines contain live, attenuated microorganisms. An attenuated virus is an active virus cultivated under conditions that disable their virulent properties or use closely related but less dangerous organisms to produce a broad immune response. Although most attenuated vaccines are viral, some are bacterial. Attenuated vaccines have some advantages and disadvantages. Attenuated (i.e., live, weakened) vaccines typically provoke more durable immunological responses, but they may not be safe for use in immunocompromised individuals. Examples of attenuated livestock vaccines include those for Bluetongue virus, lumpy skin disease virus, and foot-and-mouth virus.
- Inactivated vaccine: Vaccines that use the killed germ of a previously virulent microorganism that has been destroyed with chemicals, heat, or radiation. Inactivated vaccines may require multiple doses (booster shots) for ongoing immunity against diseases because their protection is not as strong as that of live vaccines. Examples include IPV (polio vaccine), the hepatitis A vaccine, the rabies vaccine, and most influenza vaccines.
- Toxoid vaccines: Some microorganisms can create toxins that harm cells and play a role in causing diseases. Toxoids are made from inactivated harmful substances produced by these microorganisms. Vaccines using toxoids create an immune response targeted to the toxin instead of the whole microorganism. Some toxoid vaccines include ones for tetanus and Clostridium. It is worth noting that not all toxoids come from microorganism toxins. For instance, the Crotalus atrox snake toxoid is used to vaccinate against rattlesnake bites.
- Subunit vaccines: Subunit vaccines employ specific portions of pathogens, such as isolated proteins, to trigger the immune system. This approach can also involve genetic engineering, where a gene coding for a vaccine protein is inserted into a different virus or producer cells. This yields a recombinant vaccine, such as the hepatitis B vaccine or the human papillomavirus (HPV) vaccine. For the HPV vaccine, viral proteins are expressed, forming virus-like particles (VLPs) that prompt immune responses without causing illness.
- Conjugate vaccine: Similar to recombinant vaccines, these consist of two components. They combine fragments from bacterial polysaccharide outer coats with carrier proteins, enhancing the immune response. These pieces of bacteria would be less effective alone, but when linked to a carrier protein, they generate immunity against potential infections. Conjugate vaccines are used, for instance, to protect children against pneumococcal bacterial infections.
- Outer membrane vesicles (OMVs) vaccines: OMVs are released spontaneously during growth by many groups of bacteria. They have the ability to naturally provoke an immune response in the body of a human or animal and can be manipulated to produce potent vaccines. The best-known OMVs vaccines are those developed for serotype B meningococcal disease.
- Heterologous vaccines: These are also known as "Jennerian vaccines." The heterologous vaccines contain pathogens from other animals that either do not cause disease or cause mild illness in the organism being treated. The classic example is Jenner's use of cowpox to protect against smallpox. A current example is using the vaccine made from Mycobacterium bovis to protect against tuberculosis in humans.
- Viral vector vaccines: This vaccine works by using a harmless virus to put certain genes from a harmful germ into the body. These genes help the body create specific parts of the germ, such as surface proteins. These parts then trigger the immune system to respond and protect against the germ. For instance, a recently approved experimental vaccine for deer's epizootic hemorrhagic disease uses this method along with subunit vaccine technology.
- RNA vaccine: This is a novel type of vaccine composed of nucleic acid RNA packaged within a unique delivery system such as lipid nanoparticles. The vaccine works by introducing a small piece of viral protein into the body through a piece of messenger RNA (mRNA), which prompts the immune system to produce specialized antibodies. This process does not expose individuals to the virus or result in infection. Instead, it prepares the immune system to respond quickly and effectively if the individual is exposed to the virus in the future. By providing this protection, mRNA vaccines are an important tool in the fight against disease.
Important Factors to Consider When Using Vaccines
While vaccination is an important tool in preventing disease, it cannot be relied upon solely to protect animals on the farm. It is important to understand that vaccination does not guarantee immediate immunity or resistance against all diseases. It takes time for the animal's immune system to respond to the vaccine, and several other factors will determine the level of protection provided. These include the animal's overall health, the match between the vaccine and the pathogen, and the proper administration of the vaccine. Additionally, vaccines are delicate products that must be handled and administered correctly to ensure their effectiveness. Therefore, vaccination should be viewed as just one part of a comprehensive disease prevention plan on the farm.
- Order vaccines from a trusted source. Order directly from a trusted veterinary supplier or the company producing the vaccine.
- Order an adequate amount of vaccine. Be sure to include an additional 10% when placing your order to accommodate for potential vaccine losses that might occur during the handling of animals. If feasible, opt for bottles with a lower number of doses. Keep in mind that the shelf life of each vaccine differs. While some vaccines remain effective for several hours after being mixed, others retain their efficacy for a longer duration. Avoid using a vaccine that has been open and kept in the refrigerator for extended periods. Opting for bottles with fewer doses aids in determining the necessary quantity for the day.
- Maintain correct storage conditions. Review the guidelines regarding the proper storage of the vaccine. Most animal vaccines need refrigeration within the range of 35˚F–45˚F (2˚C–7˚C). Verify the optimal functioning of your storage refrigerator, position a thermometer inside, and regularly monitor the temperature. Keep in mind that refrigerators situated in barns or open sheds may experience temperature fluctuations during the day, which can impact the vaccine's temperature. Prevent freezing or excessive warming of the vaccine at all times. Additionally, ensure that direct sunlight does not reach the vaccine.
- Observe expiration dates. Always check expiration dates, and always start using the oldest first. Once opened, make sure to mark it with the date, especially if you plan on storing it for future use.
- Follow directions for proper preparation and maintenance of vaccine shelf life after mixing. Follow the instructions provided on the bottle to guarantee the vaccine’s effectiveness. This step is of the utmost importance, because certain vaccines may need reconstitution with sterile water or the blending of components. It is essential to meticulously follow the given directions and ensure a gentle mixing process. Keep in mind that vaccines are fragile organic substances and should be shielded from temperature fluctuations. Avoid sudden temperature changes; handling a cold vaccine bottle with warm hands can swiftly alter the container's temperature and potentially impact its effectiveness.
- Avoid exposure to UV light. Do not expose vaccines to ultraviolet light from the sun. Some vaccines can be rapidly deactivated if exposed to UV light.
- Use proper injection techniques. Always inject the vaccine according to the manufacturer's directions. In animals, most vaccines are injected under the skin (subcutaneously, or SQ), intramuscular (IM), intranasally (IN; Figure 2), or intravenously (IV). Using the correct technique and location according to the species is essential. Use the right needle size and avoid reusing the same needle on another animal to reduce the risk of disease transmission. For deer, darts can be used for intramuscular (IM) injections, even though they are not ideal. When using darts, there are many variables that you need to consider. Common mistakes include missing, hitting the wrong spot, darting the same animal twice, or an incomplete dose discharge. If you are unsure whether the animal received the full dose, a second full dose is recommended. There are guidelines regarding vaccine management and administration in the livestock industry presented by the Beef Quality Assurance (BQA) program (for more information, visit https://edis.ifas.ufl.edu/an170). These best practices for vaccine management can be applied to all species, with the goal of successful vaccination and food safety.
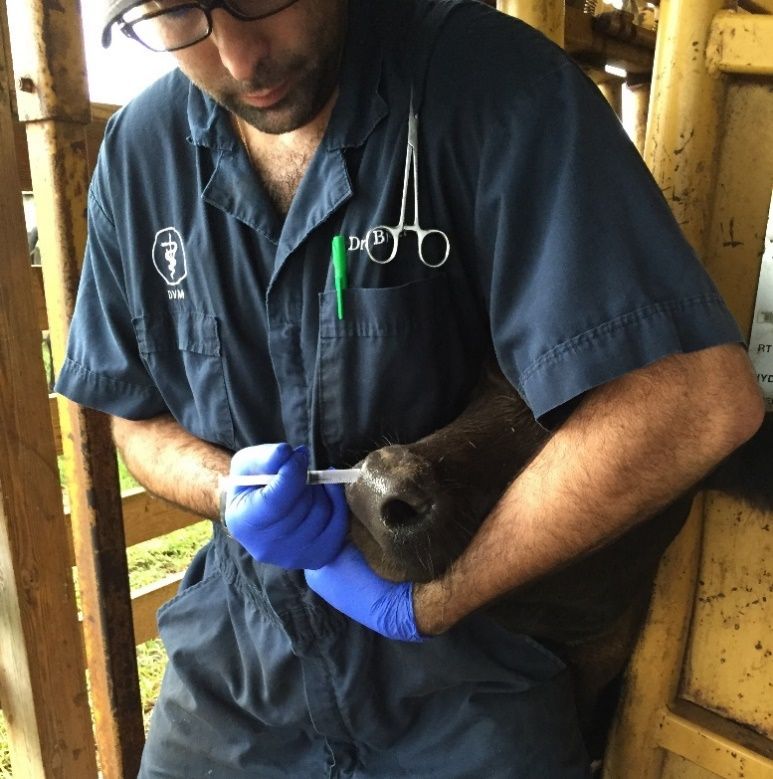
Credit: João Bittar
- Keep good records. Always record dates, animal ID, and vaccine lot number. Keeping good records is critical to improving herd health over time and may be necessary for importing or exporting animals.
- Follow regulations for correct disposal of vaccine containers. Some vaccines have products that need special disposal and that you do not want on your farm. Read the instructions for proper disposal of used containers. Regulations can vary by state.
- Maintain access to emergency information. In case of an accidental human injection or exposure to the vaccine, have the emergency number at hand for everyone working on the farm.
Conclusion
This Extension publication presented information on the basic mechanisms and types of vaccines available, as well as a few best practices for vaccine management. This information can help improve your herd health and success in your next herd vaccination. As a note, in the United States, animal vaccines are regulated by the United States Department of Agriculture (USDA, APHIS), and further information about licensed vaccines can be found on their website (https://www.aphis.usda.gov/aphis/home/).
References
Beef Checkoff. n.d. Beef Quality Assurance: National Manual. Accessed on October 21, 2021. Centennial, CO. https://www.bqa.org/Media/BQA/Docs/bqa_manual_final.pdf
CDC. n.d. “COVID-19 and Your Health.” Centers for Disease Control and Prevention. https://www.cdc.gov/index.htm
The College of Physicians of Philadelphia. n.d. “Different Types of Vaccines.” History of Vaccines. www.historyofvaccines.org
McVey, S., and J. Shi. 2010. “Vaccines in Veterinary Medicine: A Brief Review of History and Technology.” Veterinary Clinics of North America – Small Animal Practice 40 (3): 381–392. https://doi.org/10.1016/j.cvsm.2010.02.001
Meeusen, E. N. T., J. Walker, A. Peters, P.-P. Pastoret, and G. Jungersen. 2007. "Current Status of Veterinary Vaccines." Clinical Microbiology Reviews 20 (3): 489–510. https://doi.org/10.1128/CMR.00005-07
Pet Emergency Academy. n.d. “LIV- Module 3- Vaccinations.” https://www.petemergencyacademy.com/topic/liv-module-3-vaccinations/
Pollard, A. J., and E. M. Bijker. 2020. “A Guide to Vaccinology: From Basic Principles to New Developments.” Nature Reviews Immunology 21 (2): 83–100. https://doi.org/10.1038/s41577-020-00479-7
Sinha, J. K., and S. Bhattacharya. 2006. A Textbook of Immunology. Kolkata: Academic Publishers.
USDA. n.d. “Animal and Plant Health Inspection Service.” https://www.aphis.usda.gov/aphis/home/
U.S. Department of Health and Human Services. n.d. “Vaccines and Immunizations.” https://www.hhs.gov/vaccines/index.html
van der Pol, L., M. Stork, and P. van der Ley. 2015. “Outer Membrane Vesicles As Platform Vaccine Technology.” Biotechnology Journal 10 (11): 1689–1706. https://doi.org/10.1002/biot.201400395