Production Areas and Suitability
Persimmons are considered a relatively sustainable crop in Florida, rated as a 6 out of 10 on an assessment of agricultural sustainability, with a moderate commercial potential and high direct-to-consumer potential. Consumer demand would mostly be for the nonastringent varieties. Persimmons are more suitable for central and north Florida because quality and yields may be lower in the southern part of the state. Persimmons have a low chilling requirement, with success in areas that receive only 100–200 hours below 45°F (7°C), and data shows that bud dormancy may be best released at temperatures just above the normal chilling range of 32°F–45°F (0°C–7°C).
From the 2017 census of agriculture county-level data, it is clear there are two main persimmon production regions in Florida. One region is in the adjoining counties of Alachua, Levy, Marion, and Lake, which account for nearly 50 percent of the total crop acreage for the state (132 of 266 acres). Another region is in the Panhandle, in the adjoining counties of Okaloosa, Walton, and Washington, which total about 18 percent of the production acreage. There are a few other counties scattered around with more than 5 acres: Brevard (16 acres), Hernando (8 acres), and Jefferson (8 acres).
Trees grow and fruit best in central and northern Florida and can produce high yields of good-quality fruit. In south Florida, fruit quality is better with astringent types than with nonastringent types. Japanese persimmons are not listed on either the USDA Florida State Noxious Weeds List or the Florida Exotic Pest Plant Council's List of Invasive Plant Species. For information about cultivars that can be grown in Florida, visit the EDIS publication Japanese Persimmon Cultivars in Florida, available at https://edis.ifas.ufl.edu/mg242.
Propagation
Rootstock Selection
Japanese persimmons in Florida have nearly exclusively been grafted onto Diospyros virginiana, the native persimmon of the United States. D. virginiana is widely used in the southern states due to its adaptability to different soil types. It provides a tolerance of wet soils, is cold hardy, can withstand drought conditions, and is compatible with most scion cultivars. It tends to produce more suckers compared with other common rootstocks used in California, such as D. lotus and D. kaki, and can also lack uniformity and vigor. Some research completed in Florida in the 1960s comparing D. kaki and D. virginiana found little difference between the rootstocks regarding yield but did confirm the native persimmon stock promoted more suckering. D. lotus has shown some incompatibility with the main nonastringent varieties, including 'Fuyu', 'Jiro', and 'Izu', and may cause higher fruit drop in the astringent 'Hachiya'. Recent promising research from Japan on dwarfing rootstock selections, particularly of SH11, MKR1, and FDR-1, found high yield efficiency and the potential to reduce the labor requirement per tree. Fruit drop has also been shown to be inhibited in some cases by these dwarfing stocks. Future trials in Florida using different scion-rootstock combinations would aid in finding potential benefits that other rootstocks may provide.
Why Persimmons Are Grafted
Rootstocks in general have their benefits and drawbacks, and their suitability for certain soil conditions and climates can vary, so they should be chosen accordingly. Persimmons are grafted because the success rate of propagation by cuttings can be quite low, particularly hardwood shoot cuttings. Interesting research looking at micropropagated persimmon trees (own-rooted) versus grafted trees found that softwood shoot cuttings from the micropropagated trees had a notably higher average rooting percentage, 22% vs. 9%, when 3000 ppm IBA (Indole-3-butyric acid) was used for both samples. The highest successful rooting rate was found in softwood root sucker cuttings of micropropagated trees that were produced when the aboveground part of the tree was cut off. It was also noted that shorter cutting lengths of root suckers (3–4 cm; 1.2–1.6 in) displayed a higher rooting rate compared with longer lengths (25 cm; 10 in), 70% vs. 30%, respectively. Micropropagation has been known to improve cutting rooting rates due to its rejuvenation effect. It is also thought that the root suckers had better rooting rates compared to the shoots due to the root suckers being more physiologically juvenile. However, because own-rooted trees may be more vigorous than their grafted counterparts, commercial propagation by cuttings may be limited to increasing rootstock, and particularly for dwarfing rootstocks if smaller trees are desired in an orchard. Trees grafted directly onto micropropagated stocks may display negative traits, such as delayed fruiting. Thus, additional research on rootstock performance is warranted.
Propagation of Seedling Rootstocks
Fruit from local D. virginiana may be harvested, if available, when mature in the fall, and seeds can be removed and rinsed in water to remove the flesh. Seeds may be planted directly after that in potting mix if warm conditions can be provided and maintained, or the moist seeds can be treated with a fungicide and then stored in a plastic bag in a refrigerator between 35°F–45°F until planting. It is important that the seeds stay a little moist to maintain viability. Seeds and seedlings of D. virginiana may also be found through local nurseries and online sources.
Seeds may be sprouted by first placing them in moist germination paper or newspaper before planting them in potting mix, or they can be germinated in seed beds in a greenhouse in January or February. The younger seedlings can develop sunburnt leaves, so some shading early on is recommended. The seedlings are then grown out for the rest of the year and will be ready for grafting the following winter.
Scion budwood for spring grafting or budding should be collected when dormant, before bud swell, which is generally before mid-March in north Florida. Scion material can be stored in polyethylene bags at 40°F (4.4°C) for several weeks, or up to several months at 32°F (0°C), but should be checked regularly to ensure the cuttings are moist enough to prevent dehydration.
Grafting Methods
The two common methods of joining the scion and rootstock of persimmons are whip grafting and chip budding. Whip grafting can best be performed during the dormant season. Scion material with two buds provides good results, and the graft union is wrapped with tape or rubber bands. The graft can be covered in a plastic bag to keep humidity high during callusing, and waxing the cut end of the scion may also increase success.
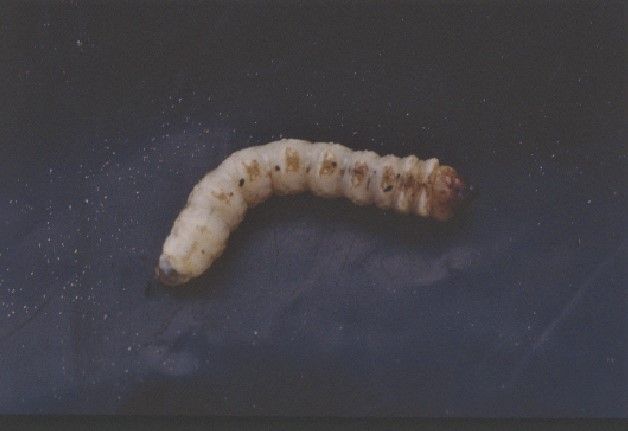
Chip budding (Figure 1) is perhaps the easiest method of propagation. When budding, the chip can be inserted in the rootstock from 1 to 6 inches above the soil line. The ideal time for spring chip budding of persimmon is when the rootstock begins growth, continuing for one month or so after leafing out. Fall chip budding can also be done if it is performed more than six weeks before the first frost to allow time to complete the callousing process. In the case of fall budding, the bud is forced in spring the following year. One key when forcing is to gradually cut the rootstock back to the point of the graft union, and not all the way until the bud has begun growing. It is important to cover the chip and bud with grafting tape to avoid it drying out. Staking newly growing shoots will help prevent them from being broken by the wind.

Credit: UF/IFAS
Other methods of propagating persimmons are used to a lesser extent or are less successful. T-budding can be done, but results may vary. Top grafting (or cleft grafting) (Figure 2) can be a way to change the cultivar being grown in an orchard but is typically performed on much older trees. If rootstocks are available, but scion bud wood happens to be limited, one temporary strategy would be to establish the rootstock in the field, and to bud or graft the scion in situ when material is available.

Credit: UF/IFAS
Field Establishment
Site Selection
The best sites for planting persimmon trees are upland sandy loam soils. Avoid low pockets to escape late-spring freeze damage after trees have begun growing. Persimmons perform best in soil pH of 6 to 7, and they are sensitive to salinity. The wood of persimmon is considered to be strong, although young shoots and branches that are weighed down with fruit can be sensitive to wind damage. In open locations, it may be necessary to establish windbreaks to lessen this problem.
Spacing
The most common training system used is the modified central leader, which in most areas, with current rootstocks, can be planted with 15 ft (4.5 m) between trees and 20 ft (6 m) between rows, allowing for 145 trees per acre. In soils that have higher or lower natural fertility, spacing can be adapted as needed to take this into account.
Initial Planting
Bare-root trees should be planted from December through February. This is done by digging a hole larger than the root system and, while keeping the graft union at or above the soil line, packing the soil around the roots until the hole is filled. Potted trees can be planted at any time of the year. However, if they are root-bound, it is best to plant them when dormant. That way the soil can be shaken off the roots, the roots can be spread out into hole and pruned if necessary, and one can avoid overstressing the tree during the growing season. Just after planting, it is important to water in the tree. A gallon or more per tree is needed, depending on the size. Maintain a weed-free zone around the tree using herbicide, shallow cultivation, or mulching.
Irrigation
Providing newly planted trees with adequate water is critical for establishment, especially in bare-root plantings, because transplanting shock can be quite a setback for young persimmons. Frequent irrigation (2 to 4 times per week) is required after planting. The quantity of irrigation will be determined by several factors, including rainfall, soil type, and evapotranspiration levels. Microsprinklers and drip irrigation are effective means to supply water and can also be used for fertigation.
As a persimmon tree matures, it will become more tolerant of occasional drought conditions, especially on the D. virginiana rootstock. It is still essential that water is available to the trees during their main growth phase in the first few months of the season. Overwatering encourages vegetative growth during the flowering and fruiting stage, and it can result in increased fruit shedding. Recent research with regulated deficit irrigation (RDI) has shown the opposite effect by reducing fruit drop when applied early in the season. However, this can cause smaller fruit at harvest time. When RDI was applied later in the season, fruit color development took place earlier. There is still more to discover on using irrigation to influence persimmon fruiting habits.
Fertilization
Small-scale demonstration or research plots with persimmons were established in 5 or more locations during the 1980s and 90s in Florida. However, these trials mostly followed common guidelines for fruit trees regarding fertilization practices, so little Florida-specific information of nutrient requirements and timing is currently known.
A general recommendation, based upon what has been done in Florida and common production practices of Japan, is listed in Table 1. It is important to note that a split fertilization plan with 3 applications is recommended, with 50% applied in late dormancy (March) and 25% each in June and September. A balanced fertilizer including macro- and micronutrients is suggested. The amount used in year 10 can be applied in succeeding years. Magnesium deficiencies can occur if soil potassium or calcium levels are too high, and manganese and iron can be scarce in soils with a pH above 7. Heavy applications of quick-release nitrogen should be avoided because this can increase fruit drop.
Training and Pruning
Modified Central Leader
This is the most common training system currently used, and it closely approaches the natural form of the tree. In this training method, a recently planted dormant tree is cut back to approximately two and a half feet, which is usually just a single stem coming from the ground. In the second winter pruning, the main central leader that grew after the first pruning is cut back by ⅓. By this time, there will be some lateral branching beginning. Select one of the laterals with a wide crotch angle growing at about the two-and-a-half-foot mark as the first main branch. Branch spacers can also be used to help widen branch angles. If present, select another branch about 8–12 inches above the first branch that is angling in another direction, and remove any branches on the trunk between the first and second. Each succeeding winter pruning continues the process of cutting the central leader back by ⅓ and selecting additional laterals every 8–12 inches to keep. The goal is to have 4–6 well-spaced branches radiating from the trunk. Once this is achieved, the central leader is then "modified" by cutting it back to the final lateral branch, which will limit significant height increases in the future (Figure 3).

Credit: UF/IFAS
Palmette System
This system requires the use of a tall trellis; however, it can double the number of trees per acre, with up to 290 trees on a 15 ft in-row spacing and 10 ft between rows. The number of horizontal wires varies, but 3 to 4 is common. The bottom wire is placed around 40 inches from the ground, with additional wires added every 3 ft. Two opposite-facing limbs in line with the trellis around 2 ft from the ground are chosen as the first branches and are attached to the trellis positioned at an upward angle of 45 degrees. Branch spreaders can also be used in this process. Moving up the centrally growing trunk, opposite-facing branch pairs every 2 ft are attached to the trellis to make the next tier of growth. Visually this system will produce a flattened vertical canopy resembling a feather. A trellis also offers the opportunity to use it as a structure for frost protection using blanket material or netting for preventing bird damage.
Other Systems
Many additional training methods exist, such as the open-centered, Y-shaped, or variations of trellis designs. Recent research investigations into a horizontal training system showed higher yield potential and reduced need for ladders due to a shorter canopy. Root zone restriction techniques can allow very high density plantings upward of 1500+ trees per acre, but long-term prospects are still unknown. When choosing a training system, cost versus benefit should always be considered.
General Pruning
Heavy pruning of persimmon is often not required or beneficial. When the main structural branches are in place, some are lightly pruned and tipped each season to stimulate more branching, while others are left unpruned. After several years of this type of pruning, the fruiting areas will get farther away from the trunk, and branch density will increase. Some of these dense areas will need to be thinned out occasionally in order to make sure light distribution within the canopy remains adequate. Any branches that do not fit the training system being used can be pruned back more or removed, and the sooner the better.
Flowering and Fruiting
Flowering
Persimmons flower and produce fruit on shoots (current season's growth) several weeks after vegetative bud break. Many of the commonly grown varieties produce female flowers (Figures 4 and 6). These occur singularly and are easily identified by the large four-lobed calyx surrounding them, which plays an important role in fruit development. Male flowers are smaller and grow in clusters on small stems (Figure 5). Persimmons can set modest crops parthenocarpically (without fertilization). However, including an occasional pollinizer tree in the planting will provide maximum yield. A ratio of 1:15 (pollinizer to main variety) was used in a demonstration trial in Florida, and although this ratio produced excessive fruit set, it can also help prevent excessive fruit drop to ensure a good crop. A ratio between 1:15 and 1:40 is suggested for Florida. 'Gailey' and 'Nishimura' are the most frequently used pollinators known for their abundance of male flowers (for more information about pollination and cultivars visit Japanese Persimmon Cultivars in Florida at https://edis.ifas.ufl.edu/mg242).

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS
Fruiting
An initial fruit drop commonly occurs following petal fall. The extent of the drop depends on multiple factors, such as previous season crop load, pollination, water and nutrient excesses, and lack of light infiltration into the canopy. Subsequent fruit drops can also occur in June and later, but they are normally lighter. Pollination lowers the amount of fruit drop, but by increasing the fruit load, more fruit thinning will be needed to bring the crop to a sustainable level, because persimmons tend toward biennial bearing if fruit load is high. Mature trees of smaller-fruited cultivars may reasonably support 250–300 fruit per tree at maturity, whereas larger-fruited cultivars could handle 150–200 fruit per tree. Trees will begin bearing fruit by the third year, with 8–10 years required for full production. Recent production data from 2013–2014 in California shows the average persimmon yield is 5.4 tons per acre. Estimates of production in south Florida are in the 3.6–5.4 tons per acre range, and north Florida is likely on the higher end. Yields are generally lower for the nonastringent varieties.
Fruit Thinning
Thinning persimmon fruit is often needed to regulate the crop load (Figure 7) and to help limit the alternate bearing tendency. Some regulation of crop load can be made through judicious pruning during the winter. Most flower buds will grow from the few end buds on a branch tip. By leaving some of these unpruned, some tipped a bud or two, and other weak branches pruned back further, less thinning of fruit is required. Hand thinning is done just after the first natural fruit drop, or about 3 weeks after flowering. Fruit should be spaced 6 inches apart on a branch to prevent rubbing damage to adjacent fruit. Remove any deformed or damaged fruit, and leave the fruit with the largest calyxes for the best fruit quality at harvest.

Credit: UF/IFAS
Harvest and Postharvest
The ripening season for persimmons varies by cultivar and runs from August through December. Nonastringent types can be picked when firm, beginning in the yellow/orange phase through full color. Astringent types should be picked at full color, when soft or nearing softness. Clip the fruit from the tree in order to prevent damage to the fruit and branches that could occur from twisting and pulling.
Nonastringent types tend to have a longer shelf life compared to astringent types, especially those that have had the astringency artificially removed. At room temperature, a nonastringent type, such as 'Fuyu', can last up to 30 days. Shelf life can be prolonged to 2 months in 0°C storage, and up to 6 months in controlled atmosphere conditions.
Bud Break and Freeze Damage
As with most fruit crops grown in areas where late winter and early spring freeze events occur, cold damage to newly growing persimmon buds and shoots can happen (Figure 8). Although persimmons lack a high chilling requirement, they do require a moderate amount of heat accumulation in order to begin growth. This makes them late to leaf out, compared with peaches, blueberries, and pears. If warm weather occurs for an extended period in January and February without frosts, growth can begin. Site selection away from low areas, maintaining proper nutrient levels, and limiting overproduction of fruit are practices to help decrease the chances of cold damage and improve the tree's tolerance to stress. If growth has begun, overhead irrigation at a rate of ¼ inch per hour to coat the tree with ice during a calm night below 32°F is also an effective protective measure. Damage can occur at the bud break stage at 27°F for a 1-hour duration. Generally, early-spring frost damage to reproductive buds of persimmon is minimized by the fact that flower buds open on new vegetative shoots after they have been growing for several weeks.

Credit: UF/IFAS
Pests and Diseases of Persimmons
Table 3 and Table 4 show the most common pests and diseases of Japanese persimmons in Florida, their symptoms, and how to control them.

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS
Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS

Credit: UF/IFAS
References
Andersen, P. C., K. R. Athearn, A. Sarkhosh, M. A. Olmstead, and J. G. Williamson. 2020. Sustainability Assessment of Fruit and Nut Crops in North Florida and North Central Florida. HS765. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/MG367
Ben-Arie, R., S. Zilkah, I. Klein, and D. Gamrasni. 2008. "Persimmon and Environment: Soil and Water Management for High Quality Fruit Production." Advances in Horticultural Science 22 (4): 286–293.
Branscome, D. 2012. White Peach Scale, Pseudaulacaspis pentagona (Targioni) (Insecta: Hemiptera: Diaspididae). EENY-076. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/IN233
Crane, J. H., and C. F. Balerdi. 1997. "Estimated Crop Yields of Tropical Fruit Crops under South Florida Conditions." https://sfyl.ifas.ufl.edu/media/sfylifasufledu/miami-dade/documents/tropical-fruit/Crop-yields-per-tree-and-acre-2010.pdf
Crocker, T. E., J. G. Williamson, and J. L. Jackson. 1996. "Demonstration Plots of Alternate Fruit and Nut Crops for Central Florida." Proceedings of the Florida State Horticultural Society 109:209–211.
Davies, F. T., R. L. Geneve, D. E. Kester, and H. T. Hartmann. 2011. Hartmann and Kester's Plant Propagation: Principles and Practice. Upper Saddle River, NJ: Prentice Hall.
Frank, S., J. Baker, and S. Bambara. 2019. "Twig Girdler." NC State Extension. https://content.ces.ncsu.edu/twig-girdler-1
George, A., B. Nissen, R. Broadley, R. Collins, P. Rigden, S. Jeffers, B. Isaacson, S. Ledger, N. Vock, and L. Chapman. 2005. Sweet Persimmon Information Kit. Agrilink, Your Growing Guide to Better Farming Guide. Agrilink Series Q105102. Brisbane, Australia: Queensland Department of Agriculture, Forestry, and Fisheries. 168.
Gomez, C., and R. F. Mizell. 2019. Green Stink Bug, Chinavia halaris (Say) (Insecta: Hemiptera: Pentatomidae). EENY-431. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/IN794
Intrigliolo, D. S., L. Bonet, E. Badal, C. Besada, and A. Salvador. 2012. "Regulated Deficit Irrigation of 'Rojo Brillante' Persimmon (Dyospyros kaki) Yield, Fruit Quality and Post-harvest Performance." VII International Symposium on Irrigation of Horticultural Crops 1038:415–422.
Jackson, D., N. E. Looney, and M. Morley-Bunker, eds. 2011. Temperate and Subtropical Fruit Production. CABI. 260–265.
Kim, W. S., S. J. Chung, K. Y. Kim, T. DeJong, and H. S. Choi. 2002. "Relationships between Ca, K and Mg Concentration and Browning of Blossom End Part of 'Fuyu' Sweet Persimmon during MA Storage." Advances in Horticultural Science 16 (2): 95–100.
Kitagawa, H., and P. Glucina. 1984. Persimmon Culture in New Zealand. Wellington, New Zealand: Science Information Publishing Center, DSIR.
Lamborn, A. R. 2017. "Persimmon." https://sfyl.ifas.ufl.edu/media/sfylifasufledu/baker/docs/pdf/horticulture/educator-resources/Persimmon.pdf
McRitchie, J. J. 1979. Cephalosporium Wilt of Persimmon. Plant Pathology Circular No. 197. Fla. Dept. Agric. & Consumer Serv. Division of Plant Industry.
Mead, F. W. 1966. Persimmon psylla. Entomology Circular No. 50. Florida Department of Agriculture, Division of Plant Industry.
Mizell, R. F., III and G. Brinen. 2015. Insect Management in Oriental Persimmon. ENY-803. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ig096
Mizell, R. F., III. 2018. Peachtree Borers in the Home and Commercial Peach Orchard. ENY-691. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/in489
Mizell, R. F., III. 2019. The Persimmon Borer Sannina uroceriformis Walker, Pest of Persimmon. ENY-835. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/in669
Mowat, A. D. 1993. "The Effect of Root Temperature on Bud Dormancy Release of Persimmon (Diospyros kaki L.)." IV International Symposium on Growing Temperate Zone Fruits in the Tropics and in the Subtropics 409:137–140.
Mowat, A. D., R. J. Collins, and A. P. George. 1993. "Cultivation of Persimmon (Diospyros kaki L.) under Tropical Conditions." IV International Symposium on Growing Temperate Zone Fruits in the Tropics and in the Subtropics 409:141–150.
Sharpe, R. H. 1966. "Persimmon Variety and Rootstock Observation." Proceedings of the Florida State Horticultural Society 79:374–378.
Tetsumura, T. 2001. "Production and Field Evaluation of Own-Rooted Trees of Japanese Persimmon (Diospyros kaki Thunb.)." PhD diss., Kyoto University.
Tetsumura, T., S. Ishimura, T. Takita, S. Funaki, H. Uchida, T. Hidaka, S. Haranoushiro, Y. Udatsu, M. Matsuo, C. Honsho, and H. Asakuma. 2019. "Tree Growth, Flowering, and Fruiting of 'Taishuu'Japanese Persimmon Grafted onto Dwarfing Rootstocks." The Horticulture Journal 88 (1): 57–66.
Tetsumura, T., R. Tao, and A. Sugiura. 2000. "Single-Node Stem Cuttings from Root Suckers to Propagate a Potentially Dwarfing Rootstock for Japanese Persimmon." HortTechnology 10 (4): 776–780.
Yakushiji, H., A. Azuma, H. Sugiura, A. Yamasaki, and Y. Koshita. 2014. "Comparison of Promising Dwarfing Rootstocks for 'Fuyu' Japanese Persimmon Trees." XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014) 1130:469–472.
Yakushiji, H., and A. Nakatsuka. 2007. "Recent Persimmon Research in Japan." Jpn. J. Plant Sci 1 (2): 42–62.
Yamada, M. 2008. "Persimmon Propagation, Orchard Planting, Training and Pruning in Japan." Advances in Horticultural Science 22 (4): 269–273.