Ginger, galangal, and turmeric (Figure 1) are emerging crops for Florida production. All of these plant species are in the Zingiberaceae family and share most aspects of their production. This publication describes production in containers or the field under Florida conditions to help guide growers interested in ginger, turmeric, and galangal production or expanding their market. All species have been evaluated by the UF/IFAS Assessment of Non-native Plants in Florida’s Natural Areas (UF/IFAS Assessment) using the Predictive Tool (an invasion risk assessment) and present a low risk of invasion in Florida (https://assessment.ifas.ufl.edu).
Plant Species
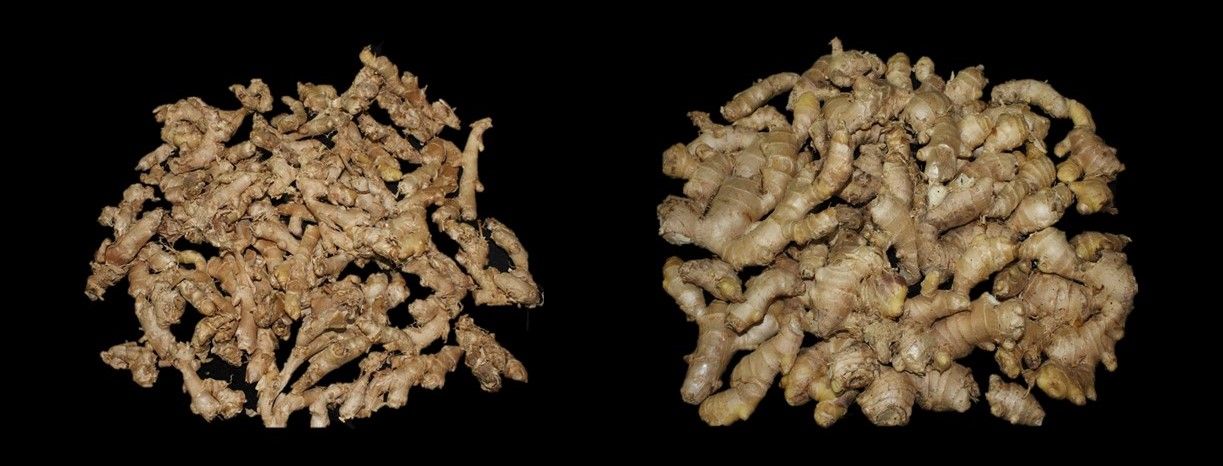
Edible ginger (Zingiber officinale, Figures 1 and 2) is traditionally used as a spice in cooking, candies, preserves, or tea and as an ingredient in beverages such as ginger ale or ginger beer. Rhizomes (Figure 2) and dried powder can be easily found in most supermarkets and ethnic grocery stores. There are approximately 150 species of Zingiber which are native to Southeast Asia. Many Zingiber species are used as ornamentals and are not edible. Edible ginger should also not be confused with non-edible ornamental gingers in other genera such as Hedychium.

Credit: Carly Nelson, UF/IFAS

Credit: Sofia Flores, UF/IFAS
Galangal (Alpinia galangal [L.] Willd., Figures 1 and 2)—also known as “galanga,” “greater galangal,” or “Thai ginger”—is another edible crop mainly cultivated in Asia (Ecocrop-FAO 1997). Sometimes used in Asian cuisine, galangal rhizomes are paler and more fibrous than ginger, and their flesh is creamy white (Chudiwal et al. 2010). There are about 230 species of Alpinia, most of them used as ornamental plants. There are three other species sometimes referred to as galangal: Alpinia officinarum, “lesser galangal”; Boesenbergia rotunda, “Chinese ginger” or “finger root”; and Kaempferia galanga, “black galangal” or “sand ginger.”
Turmeric (Curcuma longa Linn.; Figures 1, 2, and 3) is primarily used in cooking as a spice in powder form, especially in curries and masalas. Several Curcuma spp. share the common name “tumeric.” A large genus, Curcuma includes approximately 130 edible, medicinal, and ornamental species (Ravindran et al. 2007; Prasad and Aggarwal 2011). Curcuma longa is by far the most popular species: its rhizomes—which contain large amounts of bioactive compounds, such as curcuminoids—are used as a spice in cooking (Choudhary and Kumar 2018; Popuri and Pagala 2013; Ravindran et al. 2007; Ruby et al. 1995).
Commonly called “black turmeric” for its blue-black rhizomes, Curcuma caesia Roxb., is traditionally used in Asian medicine (Pandey and Chowdhury 2003; Sweetymol and Thomas 2015). (Figure 3 shows black turmeric alongside other Curcuma spp.) Curcuma amada Roxb. and Curcuma mangga Val. & Zijp. are both known as “white mango ginger” because their rhizomes have a raw mango flavor and aroma due to a high content of the volatile oil ocimene (Ayodele et al. 2018; Policegoudra et al. 2011). Curcuma zedoaria (Christm.) Rosc. (syn. C. zerumbet Roxb.) is another edible species commonly called white turmeric. Curcuma amada, mangga, and zedoaria are morphologically similar to Curcuma longa, but their rhizomes are less pungent and have a creamy-yellow color (Ravindran et al. 2007). White turmeric and white mango rhizomes are used in food and traditional Asian medicine.

Credit: Sofia Flores, UF/IFAS
Markets
United States import values of ginger and turmeric have been increasing rapidly, reflecting a rising demand. From 2013 to 2021, the annual value of rhizome ginger and turmeric imports to the United States rose from $76 million to $162 million and from $14 million to $63 million, respectively; these figures included the value of the import itself plus costs, insurance, and freight (US Census Bureau 2022). Often in demand, “baby” ginger (freshly harvested and early season) or mature rhizomes can obtain high prices of $8.00/lb. to $20.00/lb. in farmers’ markets or high-end retail outlets (Figures 2 and 4). However, Florida-grown rhizomes must compete with high quality imported products, some of which have USDA organic food certification. The retail price of imported mature rhizomes can range from $1.40/lb. to $6.00/lb.
Florida’s warm climate, demand for locally produced food and spices, multiple markets, and ease of crop production in containers or the field suggests the potential to expand production in the state. However, to profit from their ginger/turmeric harvests, local growers should avoid competing with imported bulk commodity products. This means identifying markets willing to pay a premium for local production and considering value-added processed products and niche markets such as ingredients for beverages. Ginger, galangal, and turmeric are commonly found in capsules, drinks or tonics, and teas that are sometimes labeled as herbal supplements. Traditional Asian medicine often makes use of the species’ bioactive compounds—such as the gingerols from ginger and the curcuminoids in turmeric (Ravindran et al. 2007; Gregory et al. 2008; Das et al. 2013; Smith et al. 2017; Li et al. 2016; Huang et al. 2018; Tang et al. 2018; Akter et al. 2019; Nguyen et al. 2019; Tridge 2019). Another market is for live containerized plants to home growers, with online prices often above $10.00 per plant.

Credit: Rosanna Freyre, UF/IFAS
Yield and Season Extension
Rhizomes planted in Florida fields or containers typically yield 1 to 2 pounds each. In comparison, Hawaiian-planted rhizomes typically yield over 5 pounds each; the archipelago has a longer growing season, a more moderate climate (typically 70°F to 80°F), and less change in day length because Hawaii is closer than Florida to the equator. In contrast, many parts of Florida have cool temperatures and short days in the spring and fall and very high temperatures, light, and humidity in the summer. The following points can help maximize yield and reduce risk of crop loss for Florida conditions:
- Use good quality, disease-free starting material.
- Consider the pre-sprouting of rhizomes before planting.
- Plant in a substrate or soil that is free of root diseases. Provide moderate soil moisture via irrigation and fertilize at a moderate to high rate.
- Maintain plant health by avoiding root diseases.
- Provide adequate shade to reduce stress, but do not over-shade—which may limit rhizome growth.
- Consider long-day photoperiod electric lighting and protection from cold nights in the fall.
Begin with Clean Starting Plant Material
Plants can be started from either tissue culture plants (Figure 5) or rhizomes (Figure 6) (Abbas et al. 2011; Smith et al. 2017).
Tissue culture plants are pathogen-free, initially grow more evenly than rhizomes, and are available from a Florida supplier (https://www.agristarts.com/). They produce multiple stems, which are desirable for an attractive containerized foliage plant for the home grower. However, the yield and quality of the first-year harvest from tissue culture plants of ginger and turmeric is usually lower than when planting rhizome seed pieces (Ravindran and Babu 2005; Flores et al. 2021). Rhizomes are therefore the preferred starting material for maximizing rhizome yield of ginger and turmeric. One option is to store clean rhizomes harvested from tissue culture plants in year one, and use those for planting in year two (Figure 6). The study that this publication summarizes, though, found that galangal is vigorous from tissue culture even in year one.

Credit: Carly Nelson, UF/IFAS
Credit: Sofia Flores, UF/IFAS
Sources of rhizomes for planting include specialist suppliers from Hawaii, typically around $7/lb. to $9/lb. Alternatively, imported food rhizomes can be used to plant or a grower’s own rhizomes can be kept from the previous year, but these approaches carry greater risk of pathogens. Inspect seed rhizomes closely for signs of pathogens, disease, or discoloration, and discard any suspect plants. Typical weights of each individual seed rhizome (2 to 3 inch lengths with two or more buds that will form new shoots) is about 1 to 2 ounces, meaning 12 to 16 seed rhizomes can typically be obtained per pound.
The ideal storage temperature for keeping rhizomes for seed is 55°F to 60°F at around 60% relative humidity. Growers can either (a) clean and sanitize the surface with a dip in a sanitizer such as 50 ppm of chlorine from sodium hypochlorite bleach or (b) store the rhizomes dry without washing off soil to minimize wound damage.
Rhizome fingers for planting (referred to as seeds) can be cut from larger clusters using a sanitized, non-serrated knife into individual pieces (Figure 7). The cut areas should be surface-sterilized with a sanitizing solution such as 50 ppm chlorine from sodium hypochlorite bleach or a hydrogen peroxide solution. Seed pieces should thereafter be dried (cured) for a few days before planting.

Credit: Paul Fisher, UF/IFAS
Pre-sprouting can provide an early start.
Sprouting of shoots and roots can be uneven and slow if rhizomes are planted during cool soil temperature in late winter/early spring. One strategy to lengthen the growing season is to pre-sprout rhizomes before planting outdoors (Figure 8). Seed pieces can be maintained on the surface of humid potting mix under high humidity and approximately 82°F soil temperature, with sprouting usually occurring after 1 to 2 months (Retana-Cordero et al. 2021). Avoid temperatures above 86°F and/or excess moisture to prevent rotting, and inspect each week for pathogens like Botrytis, which causes gray mold. Covering the rhizomes initially with a lightweight row cover fabric can reduce fungus gnat issues and increase humidity. Sprouted rhizomes can also be planted in small containers (up to 1 gallon) containing a pathogen-free soilless substrate to hold plants before transplanting outdoors.

Credit: Paul Fisher, Marlon Retana, and Sofia Flores, UF/IFAS
Planting, Soil, and Irrigation
Rhizomes are planted in the spring when average soil temperature is above 70°F and does not drop below 50°F during the night (March in southern Florida to May in northern Florida). Rhizomes should be planted at about 2-inches depth, whereas tissue culture plantlets should be planted at the crown.
For container production (Figure 9), a typical approach is three seed rhizomes per 15-gallon nursery container. An alternative is one rhizome per 5-gallon container. However, harvesting is more challenging, and plants are more prone to being blown over in wind. Plants produce fairly shallow roots, and an 8-inch depth of potting mix is therefore adequate. More details can be found in the study by Retana-Cordero et al. (2022a). Although growers in some areas mound the soil over the rhizomes during the growing season, the authors of this publication have not found this beneficial in Florida because of the state’s shorter growing season. Use a substrate (typically with components such as coconut coir, peat, bark, wood fiber, or perlite) that has coarse particles for adequate drainage and aeration; these particles simplify the cleaning of rhizomes at the end of the season while still providing adequate water retention.
For in-ground production, plant at a 1.5 foot spacing along rows either in a well-drained soil or in soilless substrate in prepared beds (Figure 9). Straw or plastic mulch can be used to improve weed control in field soil, long-term crop production. If using plastic, ensure planting holes are large enough to allow new shoots to emerge.
Plants have a moderate to high fertilizer requirement. This publication’s authors have grown crops successfully with:
- three applications (at planting and two subsequent applications) with an organic poultry-based granular 7-5-10 (N-P2O5-K2O) fertilizer at 100 grams (3.3 oz.) per plant,
- controlled release 8 to 9 month fertilizer at planting with 120 grams (4 oz.) per plant, and
- water-soluble fertilizer such a 15-5-15 at 100 ppm nitrogen with each irrigation.
No plant nutrient requirement recommendations for these crops are currently available from UF/IFAS, and these plant nutrient rates are for guidance only. Avoid excess salt levels in soil because excess salt exacerbates drought stress. Avoid fertilization in the winter when plants go dormant and are not actively taking up nutrients.

Credit: Marlon Retana and Paul Fisher, UF/IFAS
Once established in the summer, plants typically require either irrigation or rainfall each day. Plants that are too dry will initially show leaf curling (Figure 10) and quickly thereafter symptoms of tip or marginal burn and leaf yellowing. Irrigation can be stopped a month before harvest in the winter because plants are not actively taking up water; doing so makes it easier to remove dry soil from the rhizomes during harvest.
Avoid biotic stresses, especially root pathogens.
The most common pathogen issue is disease of the roots, rhizomes, and vascular tissue caused by a range of pathogens in the soil, equipment, or starting plant material. Sanitation and scouting are therefore critical.
Avoid waterlogging because plants, particularly ginger, are very susceptible to root pathogens. The UF/IFAS Plant Diagnostic Center has identified Ralstonia solanacearum, Pythium spp., Phytophthora spp., Fusarium oxysporum, and root-knot nematodes on Florida-grown ginger and turmeric rhizomes and roots.
Plants with rotten rhizomes should be disposed of to reduce pathogen spread. Any rotten plant material, potting mix, or soil should not be used for future crops. Equipment used with infected plants should be sanitized before reuse. If land is available, use crop rotation with crops that are not hosts of the established pathogens to reduce initial inoculum. Plant rhizome crops in the same field only after a long-term crop rotation (i.e., every 3 to 5 years).

Credit: Paul Fisher, Carly Nelson, and Nathalia Tello, UF/IFAS
These crops are not particularly prone to pressure from insect and mite pests, and the publication’s authors have not observed crop losses or the need to spray insecticides in Florida under high tunnel or field conditions. Minor damage from army worms and grasshoppers was observed outdoors. When grown in greenhouses, fungus gnats, aphids, and spider mites can also impact plant health. Under greenhouse conditions the insect and mite pests are manageable; farmers should use commercially available natural biological controls.
Provide adequate shade.
Ginger and galangal are cultivated in tropical and subtropical environments, including India, China, Nigeria, Indonesia, Bangladesh, Australia, and Hawaii in the U.S. Optimum growing temperatures are in the range of 70°F to 85°F. Low temperatures lead to slow growth and dormancy. High temperatures increase heat stress, especially when plants are in high light or soil is too saline or not moist enough.
Ginger and galangal thus require 25% to 40% shade in Florida during the summer, especially during crop establishment, to avoid heat stress and foliar damage (Figure 11). Over-shading will result in vigorous and healthy top growth but low rhizome yield because of reduced photosynthesis. Shading options include:
- Growing under a shade structure, with or without plastic rain cover to control soil moisture.
- Growing in a naturally shaded area or possibly interplanting with a tall crop between rows.
- Spraying kaolin sprays onto foliage every two weeks, especially during crop establishment. Kaolin is a clay material that physically blocks sunlight. UF/IFAS research by Retana et al. (2022b) found 87% greater ginger yield and 47% greater turmeric yield with biweekly sprays of kaolin for the first 8 weeks compared with the unsprayed control plants in an open field planting. No differences in yield were measured between biweekly kaolin sprays during the entire cycle (20 weeks) and sprays applied only during the first 8 weeks, demonstrating the need to avoid stress immediately after planting. Though effective, kaolin can be washed off with summer thunderstorms.

Credit: Paul Fisher, UF/IFAS
Lengthen the daylight period in the fall.
As the temperature gets colder and the days grow shorter in the fall, leaves turn yellow, plants naturally enter dormancy; and rhizomes enlarge and become more fibrous. Tunnel greenhouses with roll-up sides can help north Floridian growers extend their plant season. Growers can roll down the sides in the colder months of spring and fall, protecting their plants. Electric lights can also increase the season for harvest of fresh rhizomes, which may result in higher sale prices and yield.
Ginger and turmeric are quantitative short-day plants for rhizome growth. Ginger requires a long day photoperiod (≥ 12 hours) for continuous vegetative growth of shoots and leaves without entering dormancy. Rhizome production reportedly favors short day lengths (≤ 11 hours) (Adaniya et al. 1989; Pandey et al. 1996). However, research at UF/IFAS showed that increasing the duration of long days increased yield regardless of whether plants finished under short days (Flores et al. 2021).
In contrast, galangal has not shown sensitivity to photoperiod-induced dormancy. Galangal flowers during the summer in the subtropics but can produce flowers and rhizomes all year in tropical areas regardless of the photoperiod (Tang et al. 2018).
Day length can be extended with electric lights strung above the plants either after dusk or before dawn to a total of at least 14 hours, or by providing a night interruption period between 10 pm and 2 am. This can be achieved with energy-efficient LED lamps to provide a low light level (minimum of 2 micromol.m-2.s-1 of photosynthetically active radiation, Figure 12). If temperatures remain above about 50°F, plants remain vegetative and keep actively growing, resulting in higher rhizome yield (Figure 13).

Credit: Sofia Flores, UF/IFAS

Credit: Sofia Flores, UF/IFAS
Harvest Maturity
Harvest time after planting depends on the end use. Five months after planting (fall harvest) is enough for ginger rhizomes that will be sold as fresh early-season “baby” ginger. Baby ginger has low fiber and pungency and is sometimes sold with short segments of green stem attached (Figures 4 and 14). Ginger and turmeric rhizomes harvested approximately seven months after planting (i.e., winter harvest) are suitable for curing and selling in retail and as an ingredient for making preserves. Rhizomes with longer growing periods are more suitable to be dried or used to extract essential oils (Ravindranan and Babu 2005). Irrigation can be stopped before harvest to produce maximum yield of mature rhizomes and facilitate cleaning.

Credit: Paul Fisher, UF/IFAS
Galangal is also sold fresh, in slices as preserves, or dried in powder form. As with ginger, galangal rhizomes harvested for seed are likely to have cut ends, which must be healed and dry, and should not be soft or moldy. Rhizomes of galangal develop quickly and for fresh produce they should be harvested approximately four months after planting, otherwise they become very fibrous. However, for essential oil extraction, rhizomes are harvested after approximately seven months of growth (Ochatt and Jain 2007). The shelf life of galangal rhizomes is limited by browning of the cut surface, therefore they are usually treated with anti-browning solutions prior to marketing (Chinwong et al. 2006).
Harvest and Postharvest Handling
Follow good agricultural practices for harvest and postharvest phases. (See EDIS Publication #FSHN06-01.)
Harvest and handling guidelines are similar for ginger, galangal, and turmeric. Rhizomes can be hand harvested using a modified potato digger; larger operations can lift them out of the ground using a tractor-mounted cutter bar (Nishima et al. n. d.).
After harvest, rhizomes should be washed with water to remove the soil, and stems and roots should be trimmed off. This is a labor-intensive process if done manually, and automated root washing equipment is strongly recommended. Treat the surface of the rhizomes with a sanitizing solution—such as 50 ppm chlorine from sodium hypochlorite bleach—or a hydrogen peroxide product for disinfection. Curing of ginger rhizomes for three to five days under ambient conditions toughens the skin and reduces weight loss during storage. Refrigerated storage at 54°F to 57°F and 85% relative humidity will minimize excessive drying; however lower temperatures will cause chilling injury (Paul and Chen 2015). Any diseased rhizomes that develop during storage should be discarded. Properly prepared and stored rhizomes should have up to 90 days of storage. (Again, for more on how to follow good agricultural practices for harvest and postharvest phases, visit EDIS publication #FSHN06-01.)
Acknowledgements
We thank our industry partners in the Floriculture Research Alliance (FloricultureAlliance.org), the SEEDIT Research/Extension funding program from UF/IFAS, the UF Field and Fork Program, and the FDACS Specialty Crops Block Grant program for their support. We thank AgriStarts, Just Ginger Florida, and hawaiianorganicginger.com for supplying plant materials.
References
Abbas, M. S., H. S. Taha, U. I. Aly, H. M. El-Shabrawi, and E. I. Gaber. 2011. “In vitro propagation of ginger (Zingiber officinale Rosco).” Journal of Genetic Engineering and Biotechnology 9 (2):165–172. https://doi.org/10.1016/j.jgeb.2011.11.002
Adaniya, S., M. Shoda, and K. Fujieda. 1989. “Effect of Day Length on Flowering and Rhizome Swelling in Ginger (Zingiber officinale Roscoe). Journal of the Japanese Society for Horticultural Science 58 (3):649–656. https://doi.org/10.2503/jjshs.58.649
Akter, J., Md. A. Hossain, K. Takara, Md. Z. Islam, and D. Hou. 2019. “Antioxidant Activity of Different Species and Varieties of Turmeric (Curcuma spp): Isolation of Active Compounds.” Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 215:9–17. https://doi.org/10.1016/j.cbpc.2018.09.002
Ayodele, V. O., O. M. Olowe, C. G. Afolabi, and I. A. Kehinde. 2018. “Identification, Assessment of Diseases and Agronomic Parameters of Curcuma amada Roxb (Mango Ginger).” Current Plant Biology 15:51–57. https://doi.org/10.1016/j.cpb.2018.10.001
Chinwong, U., J. Siriphanich, and R. Chaiwat. 2006. “Quality of Fresh-Cut Galangal Harvested at Different Maturity Stages.” Unpublished manuscript. https://www.phtnet.org/download/phtic-seminar/466.pdf
Choudhary, V. K., and P. S. Kumar. 2018. “Weed Suppression, Nutrient Leaching, Water Use and Yield Of Turmeric (Curcuma longa L.) under Different Land Configurations and Mulches.” Journal of Cleaner Production 210: 795–803. https://doi.org/10.1016/j.jclepro.2018.11.071
Chudiwal, A. K., D. P. Jain, and R. S. Somani. 2010. “Alpinia galanga Willd.—an overview on Phyto-Pharmacological Properties.” Indian Journal of Natural Products and Resources 1 (2):143–149. https://nopr.niscpr.res.in/bitstream/123456789/9821/1/IJNPR%201(2)%20143-149.pdf
Das, S., P. Mondal, and Md. Kamaruz Zaman. 2013. “Curcuma caesia Roxb. and Its Medicinal Uses: a Review.” International Journal of Research in Pharmacy and Chemistry 3 (2): 370–375. http://www.ijrpc.com/files/29-3103.pdf
Ecocrop-FAO (Encrop code #3052; 1997). Alpinia galanga. https://gaez.fao.org/pages/ecocrop-find-plant
Flores, S., M. Retana-Cordero, P. R. Fisher, R. Freyre, and C. Gómez. 2021. “Effect of Photoperiod, Propagative Material, and Production Period on Greenhouse-Grown Ginger and Turmeric Plants.” HortScience 56 (12): 1476–1485. https://doi.org/10.21273/HORTSCI16025-21
Gregory, P. J., M. Sperry, and A. F. Wilson. 2008. “Dietary Supplements for Osteoarthritis.” American Family Physician 77 (2):177–184. https://pubmed.ncbi.nlm.nih.gov/18246887/
Huang, G. C., C. L. Kao, W. J. Li, S. T. Huang, H. T. Li, and C. Y. Chen. 2018. “A New Phenylalkanoid from the Rhizomes of Alpinia galanga.” Chemistry of Natural Compounds 54: 1072–1075. https://doi.org/10.1007/s10600-018-2558-x
Li, Y., Y. Hong, Y. Han, Y. Wang, and L. Xia. 2016. “Chemical Characterization and Antioxidant Activities Comparison in Fresh, Dried, Stir-Frying and Carbonized Ginger.” Journal of Chromatography B 1011: 223–232. https://doi.org/10.1016/j.jchromb.2016.01.009
Nguyen, L., L. T. Duong, and R. S. Mentreddy. 2019. “The U.S. Import Demand for Spices and Herbs by Differentiated Sources.” Journal of Applied Research on Medicinal Aromatic Plants 12: 13–20. https://doi.org/10.1016/j.jarmap.2018.12.001
Nishina, M. S., D. M. Sato, W. T. Nishijima, and R. F. L. Mau. n.d. “Ginger Root Production in Hawaii.” University of Hawai’i at Manoa. Commodity Fact Sheet GIN-3(A). https://gms.ctahr.hawaii.edu/gs/handler/getmedia.ashx?moid=3271&dt=3&g=12
Ochatt, S., and S. M. Jain. 2007. Breeding of Neglected and Under-Utilized Crops, Spices, and Herbs. Boca Raton: CRC Press. https://doi.org/10.1201/9781482280548
Pandey, A. K., and A. R. Chowdhury. 2003. “Volatile Constituents of the Rhizome Oil of Curcuma caesia Roxb. from central India.” Flavour and Fragrance Journal 18: 463–465. https://doi.org/10.1002/ffj.1255
Pandey, Y. R., C. Sagwansupyakorn, O. Sahavacharin, and N. Thaveechai. 1996. “Influence of Photoperiods on Dormancy and Rhizome Formation of Ginger (Zingiber officinale Roscoe).” Kasetsart Journal – Natural Science 30: 386–391.
Paul, R. E., and C. C. Chen. 2015. “Ginger Postharvest Quality-Maintenance Guidelines.” University of Hawaii at Manoa. VC-2.
Policegoudra, R. S., S. M. Aradhya, and L. Singh. 2011. “Mango Ginger (Curcuma amada Roxb.)—a Promising Spice for Phytochemicals and Biological Activities.” Journal of Biosciences 36: 739–748. https://doi.org/10.1007/s12038-011-9106-1
Popuri, A. K., and B. Pagala. 2013. “Extraction of Curcumin from Turmeric Roots.” International Journal of Innovative Research & Studies 2: 289–299. https://www.researchgate.net/file.PostFileLoader.html?id=54246744d5a3f2ab2f8b469e&assetKey=AS%3A273559857893418%401442233160841
Prasad, S., and B. B Aggarwal. 2011. “Turmeric the Golden Spice: from Traditional Medicine to Modern Medicine.” In Herbal Medicine: Biomolecular and Clinical Aspects, edited by I. F. F. Benzie and S. Wachtel-Galor. 2nd ed. Boca Raton: CRC Press/Taylor & Francis. https://doi.org/10.1201/b10787
Ravindran, P. N. and K. N. Babu. 2005. Ginger: the Genus Zingiber. Boca Raton: CRC Press.
Ravindran, P. N., K. N. Babu, and K. Sivaraman. 2007. Turmeric: the Genus Curcuma. Boca Raton: CRC Press. https://doi.org/10.1201/9781420006322
Retana-Cordero, M., S. J. Flores, P. R. Fisher, R. Freyre, and C. Gómez. 2022a. “Effect of Container Volume and Planting Density on Ginger and Turmeric Growth and Yield.” HortTechnology 32 (5): 425–434. https://doi.org/10.21273/HORTTECH05092-22
Retana-Cordero, M., P. R. Fisher, and C. Gómez. 2021. “Modeling the effect of temperature on ginger and turmeric rhizome sprouting.” Agronomy 11 (10): 1931. https://doi.org/10.3390/agronomy11101931
Retana-Cordero, M., S. Flores, R. Freyre, and C. Gómez. 2022b. “Strategies to Reduce Radiation Stress in Open-Field Ginger and Turmeric Production.” Agronomy 12 (8): 1910. https://doi.org/10.3390/agronomy12081910
Ruby, A. J., G. Kuttan, K. D. Babu, K. N. Rajasekharan, and R. Kuttan. 1995. “Anti-Tumor and Antioxidant Activity of Natural Curcuminoids.” Cancer Letters 94 (1): 79–83. https://doi.org/10.1016/0304-3835(95)03827-J
Smith, T., K. Kawa, V. Eckl, C. Morton, and R. Stredney. 2017. “Herbal Supplement Sales in US Increase 7.7% in 2016.” Market Report 115: 56–65. http://herbnutritionals.com/wp-content/uploads/2017/12/Herbal-Supplement-Sales-in-US-Increase-7.7-in-2016.pdf
Sweetymol, J. and T. D. Thomas. 2015. “High Frequency Callus Organogenesis, Large Scale Cultivation and Assessment of Clonal Fidelity of Regenerated Plants of Curcuma caesia Roxb., an Important Source of Camphor.” Agroforestry Systems 89 (5): 779–778. https://doi.org/10.1007/s10457-015-9811-0
Tang, X., C. Xu, Y. Yagiz, A. Simonne, and M. R. Marshall. 2018. “Phytochemical Profiles, and Antimicrobial and Antioxidant Activities of Greater Galangal [Alpinia galangal (Linn.) Swartz.] Flowers.” Food Chemistry 255: 300–308. https://doi.org/10.1016/j.foodchem.2018.02.027
Tridge (HS Code #091030; 2019). Intelligence, Turmeric. Overview of Global Turmeric Trade. https://www.tridge.com/intelligences/turmeric1
US Census Bureau: Economic Indicators Division USA Trade Online. 2022. U.S. Import and Export Merchandise Trade Statistics. https://usatrade.census.gov/