Introduction
Pomegranate (Punica granatum L.) was introduced to Florida and the southeastern United States in the 1700s. Since its introduction, it has primarily been utilized as a backyard shrub or tree, with little commercial production in Florida or neighboring states. However, over the past 15 years, the potential of pomegranate as an alternative fruit crop has drawn the attention of growers, nurseries, researchers, and Extension agents. Early field trials indicated that pomegranates are highly susceptible to several fungal pathogens that are common in Florida due to the state’s humid subtropical climate. One of these fungal pathogens is Colletotrichum gloeosporioides, known to cause severe anthracnose fruit rot in pomegranates. Although several fungicides have been studied, with a few approved for anthracnose control in Florida, a more sustainable and cost-effective strategy is the use of anthracnose-resistant cultivars. Such cultivars will be valuable not only for commercial production but also for breeding new disease-resistant cultivars. This publication presents our findings from evaluating 35 pomegranate cultivars under natural disease pressure in central Florida and by artificial inoculation using a common isolate of C. gloeosporioides (Schaller et al. 2023). We further validated anthracnose resistance by inoculating detached mature fruit. This publication is prepared for those interested in pomegranate resistance to major fungal diseases in the southeast United States.
Anthracnose Fruit Rot—Symptoms and Impact
Anthracnose, caused by various Colletotrichum species, poses a significant threat to numerous fruit crops. Xavier et al. (2019) found that anthracnose is the most important disease in pomegranate, leading to severe fruit rot and substantial yield losses. Colletotrichum spores, being waterborne, typically initiate infection at the start of the rainy season, which occurs from late May to June through September in Florida. Anthracnose on pomegranate fruit is characterized by sunken lesions, and it can cause complete rot of the fruit, both externally and internally (Figure 1). In Florida, anthracnose disease pressure can be so intense that pomegranate fruit succumbs to rot well before maturity, resulting in up to 100% fruit loss.

Credit: Alexander Schaller, UF/IFAS
Anthracnose Severity in Field Evaluation under Natural Infection
Field evaluations were carried out at the UF/IFAS Gulf Coast Research and Education Center in central Florida, with an experimental pomegranate orchard established in 2015. The pomegranate plants had reached six years of age at the commencement of the field evaluation. Prior to each evaluation season, pomegranate plants were defoliated with ethephon, a plant growth regulator often used to promote fruit ripening and abscission. No fungicides were applied to these plants during the evaluations. Thirty-five pomegranate cultivars were included in the evaluation in 2021. In 2022, some trees did not produce enough fruit, so only 27 cultivars were included in the evaluation. These cultivars originated from five different regions of the world: southeastern United States (Florida and Georgia), western United States (California), Turkmenistan and its adjacent region, the former Soviet Union, and India. To assess fruit rot severity under natural disease pressure, young fruit were selected arbitrarily and tagged in May 2021 and May 2022. Fruit was examined weekly for eight weeks from May to July. When fruit rot symptoms were first noticed, the size of the rotted area (lesion size) on each fruit was measured, and then the lesion size measurements were converted to a 0 to 6 rating scale:
- 0—for no anthracnose lesions
- 1—for a lesion only on the calyx
- 2—lesions measuring up to 2 cm in diameter
- 3—lesions larger than 2 cm but up to 4 cm
- 4—larger than 4 cm but up to 6 cm
- 5—lesions larger than 6 cm
- 6—extensive fruit rot that results in fruit drop.
In these evaluations, anthracnose symptoms began to appear on young fruit in May in both years, and fruit rot lesions continued to enlarge as the fruit developed. Several patterns of disease development were observed in both years (Figure 2). For the cultivars that were highly susceptible to anthracnose, the disease symptoms initially appeared in the early fruit development stage, progressed almost linearly, and rapidly increased over the season with the onset of the rainy season. For resistant cultivars, disease progressed at a much slower pace and did not expand significantly on the fruit. Some cultivars, including ‘Eversweet’, ‘Girkanets’, and ‘Parfianka’, showed little disease in the early phase but experienced a sharp increase in disease symptoms later in the season (Figure 2). At the end of the evaluation in 2021, ‘Azadi’, ‘Arakta’, and ‘Fleishman’ had the least fruit rot, whereas ‘Kazake’, ‘Desertnyi’, and ‘Eve’ had the most fruit rot (Figure 2). At the end of the evaluation in 2022, ‘Azadi’, ‘Fleishman’, and ‘Arakta’ again had the least fruit rot, whereas ‘Kazake’, ‘Eve’, and ‘Al-Sirin-Nar’ had the most fruit rot (Table 1).
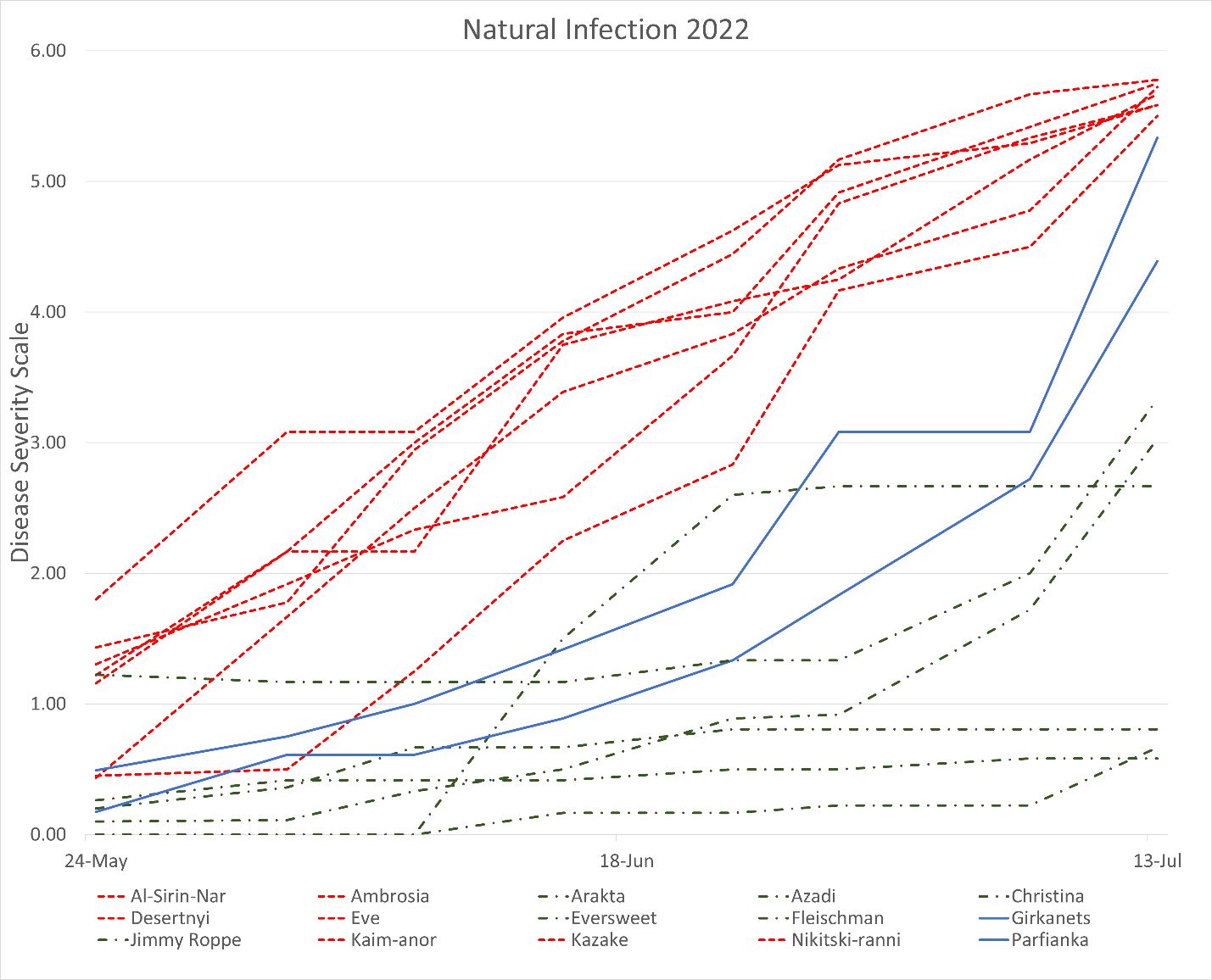
Credit: Alexander Schaller, UF/IFAS
Anthracnose Severity after Artificial Spore Inoculation
Colletotrichum gloeosporioides isolate C30 was used to inoculate pomegranate fruit, as described by Schaller et al. (2023). All inoculated fruit were examined for fruit rot lesions every week for eight weeks, and the size of each lesion was measured. Lesion measurements were then converted to ratings from 0 to 6 on a disease-severity scale.
In 2021 and 2022, fruit rot progressed almost linearly on inoculated fruit for most of the cultivars. A small number of cultivars, including ‘Eversweet’ and ‘Girkanets’, experienced a sharp increase in disease symptoms towards the end of the evaluation period (Figure 3). Pomegranate cultivars showed significant differences in disease severity in 2021 and 2022. The cultivars ‘Azadi’, ‘Fleishman’, and ‘Surh-Anor’ had the least fruit rot in 2021, whereas ‘Kazake’, ‘Molla Nepes’, and ‘Ambrosia’ had the most fruit rot. In 2022, ‘Azadi’, ‘Christina’, and ‘Eversweet’ had the least fruit rot in 2022, while ‘Eve’, ‘Al-Sirin-Nar’, and ‘Kazake’ had the most fruit rot.
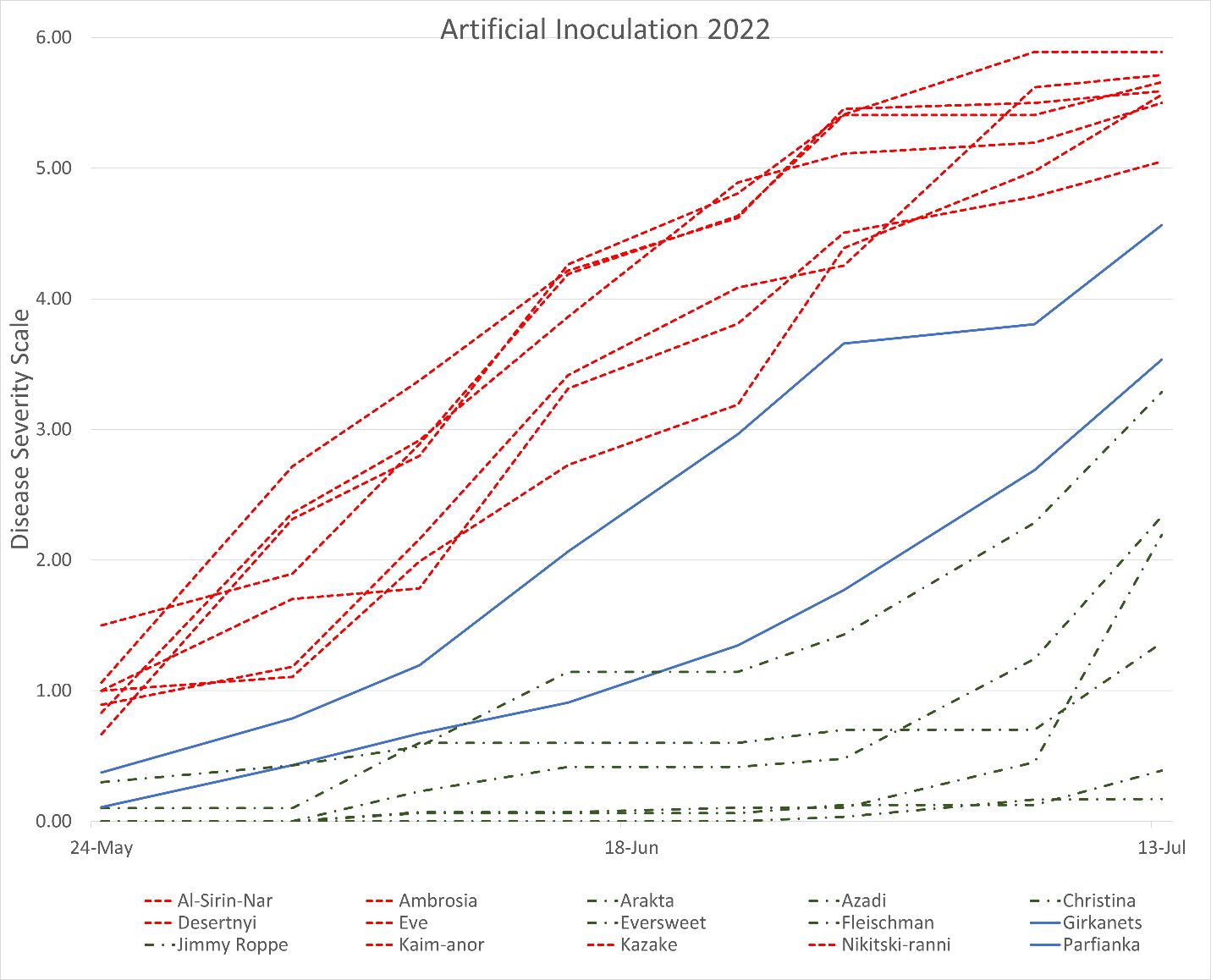
Credit: Alexander Schaller, UF/IFAS
Table 1. Average fruit rot severity rating for fruit that were naturally infected or artificially inoculated for 35 pomegranate cultivars.
Ranking Pomegranate Cultivars for Resistance to Anthracnose Fruit Rot
Based on anthracnose severity ratings from natural infection and artificial inoculation over two years and the percent of fruit that had large lesions (level 5 or 6 on the aforementioned rating scale), we ranked 27 pomegranate cultivars into five resistance categories against anthracnose. One cultivar, ‘Azadi’, displayed a high level of resistance. Additionally, we identified five as resistant, eight as moderately resistant, six as susceptible, and seven as highly susceptible (Table 2). Eight cultivars didn’t produce sufficient numbers of fruit for evaluation in 2022 and lacked complete data for both artificial inoculation and natural infection over the two-year period, and therefore, they were not included in the ranking process.
Among the cultivars exhibiting resistance to anthracnose, it is noteworthy that all except ‘Arakta’ showed common characteristics of having yellow to light pink peel and light pink arils (the fleshy, transparent tissue that covers pomegranate seeds). Further investigations are required to determine whether pomegranate fruit peel or aril color is associated with anthracnose resistance.
The six cultivars demonstrating resistance to anthracnose originate from diverse regions. ‘Christina’ and ‘Jimmy Roppe’ are from Florida/Georgia, ‘Eversweet’ and ‘Fleishman’ from California, ‘Arakta’ from India, and ‘Azadi’ from Turkmenistan. Of particular interest is the observation that ‘Azadi’ has evolved resistance to anthracnose despite being cultivated in an area where the disease is not commonly encountered.
Table 2. Resistance of 27 pomegranate cultivars to anthracnose fruit rot caused by C. gloeosporioides.
Expression of Anthracnose Resistance on Detached Mature Fruit
Mature fruit from seven cultivars (‘Afganski’, ‘Kazake’, ‘Eversweet’, ‘Nikiski Ranni’, ‘Al-Sirin-Nar’, ‘Fleishman’, and ‘Azadi’) varying in their susceptibility to anthracnose were sourced from the National Clonal Germplasm Repository in Davis, California. These fruits were inoculated with C. gloeosporioides spores and incubated in a high-humidity environment for 14 days. ‘Azadi’ mature fruit exhibited minimal lesions and showed strong resistance to anthracnose (Figure 4). Conversely, mature fruit of ‘Afganski’ and ‘Kazake’ displayed large lesions and high levels of susceptibility to anthracnose. These results may suggest a promising approach for anthracnose resistance screening in pomegranate cultivars and breeding lines. The method may involve inoculating and incubating detached mature fruit in a laboratory setting over a two-week period. This alternative approach is more efficient and controlled compared to the traditional method of inoculating open flowers or young fruitlets in the field and observing the inoculated fruit over two months.
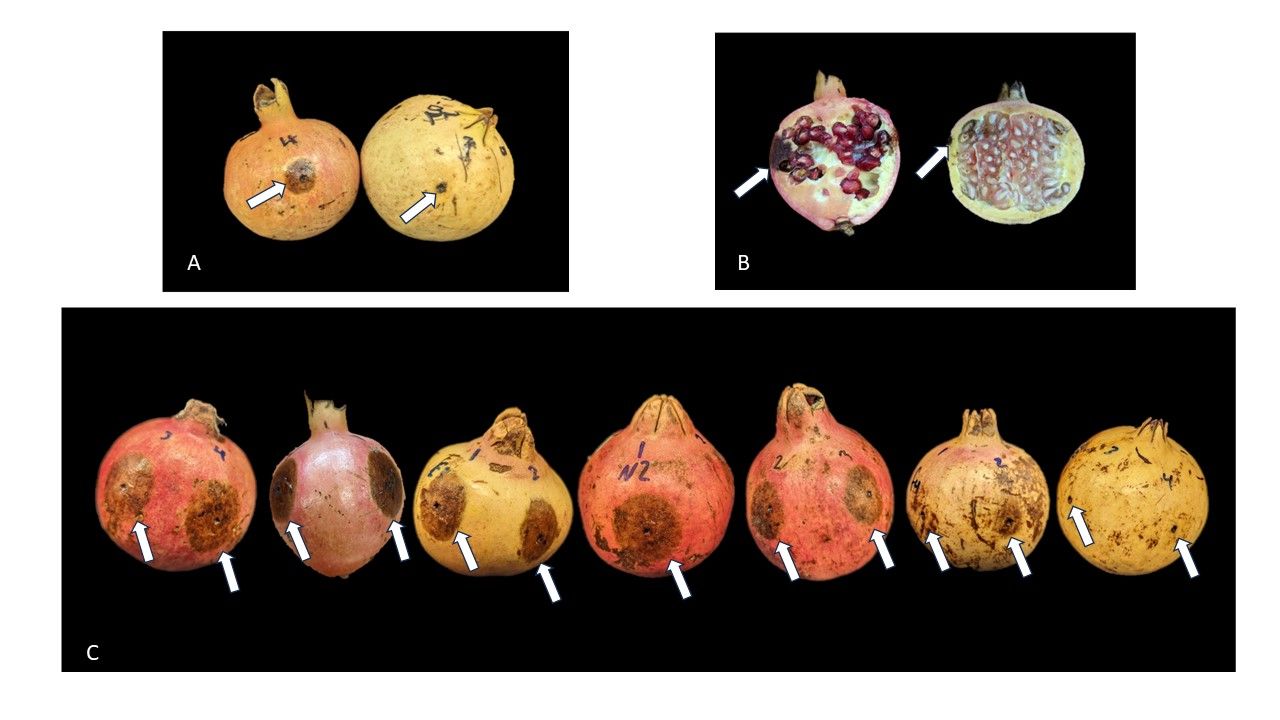
Credit: Alexander Schaller, UF/IFAS
Conclusion
Anthracnose represents a significant threat to pomegranate crops, resulting in severe fruit damage and substantial yield losses. This threat is particularly pronounced in Florida and the southeastern United States, where the disease thrives. In our study, we have successfully identified six cultivars, namely ‘Azadi’, ‘Araka’, ‘Christina’, ‘Eversweet’, ‘Fleishman’, and ‘Jimmy Roppe’, that exhibit resistance to this debilitating disease. The discovery of these cultivars warrants further horticultural trials for cultivation in Florida. Moreover, identifying these resistant cultivars opens exciting prospects for developing new pomegranate cultivars with strong resistance to anthracnose for Florida and other regions where anthracnose fruit rot is prevalent.
Acknowledgment
This project was supported by the USDA AMS Multistate Specialty Crop Block Grant through the California Department of Food and Agriculture (19-1043-002-SF), the AMS Specialty Crop Block Grant through the FDACS Specialty Crop Block Grant Program (#00099169), and the former Florida Pomegranate Association. Florida growers, C. Weistein, R. Bonsteel, J. Alexander, and D. Bice, donated plant materials.
References
Deng, Z., W. Castle, G. E. Vallad, S. Agehara, M. Thetford, and J. C. Dı́az-Pérez. 2019. “Pomegranate: An Emerging Fruit Crop in Southeast United States?” ISHS Acta Horticulturae 1254: 149–156. https://doi.org/10.17660/ActaHortic.2019.1254.23.
LaRue, J. H. 1980. “Growing Pomegranates in California.” University of California Division of Agricultural Sciences, Leaflet 2459. Archived on April 3, 2014. https://web.archive.org/web/20140403201020/http://ucanr.edu/sites/Pomegranates/files/122804.pdf
Mangandi, J., N. A. Peres, and V. M. Whitaker. 2015. “Identifying Resistance to Crown Rot Caused by Colletotrichum Gloeosporioides in Strawberry.” Plant Disease 99 (7): 954–961. https://doi.org/10.1094/PDIS-09-14-0907-RE
Phillips, D. A., P. F. Harmon, J. W. Olmstead, N. A. Peres, and P. R. Munoz. 2018. “Screening for Susceptibility to Anthracnose Stem Lesions in Southern Highbush Blueberry.” HortScience 53 (7): 920–924. https://doi.org/10.21273/HORTSCI12994-18
Schaller, A., J. M. Chater, G. E. Vallad, J. Moersfelder, C. Heinitz, and Z. Deng. 2023. “Pomegranate cultivars with diverse origins exhibit strong resistance to anthracnose fruit rot caused by Colletotrichum gloeosporioides, a major disease in southeast United States.” Horticulturae 9 (10): 1097. https://doi.org/10.3390/horticulturae9101097
Stover, E., and E. W. Mercure. 2007. “The Pomegranate: A New Look at the Fruit of Paradise.” HortScience 42 (5): 1088–1092. https://doi.org/10.21273/HORTSCI.42.5.1088
Xavier, K. V., A. N. KC, and G. E. Vallad. 2020. “Fungicide Application Timing Essential for the Management of Leaf Spot and Fruit Rot on Pomegranate (Punica granatum L.) in Florida.” Plant Disease 104 (6): 1629–1637. https://doi.org/10.1094/PDIS-10-19-2224-RE
Xavier, K. V., A. N. KC, and G. E. Vallad. 2019. “Diseases of Pomegranate (Punica granatum) in Florida: PP349, 10/2019.” EDIS 2019 (5): 5. https://doi.org/10.32473/edis-pp349-2019
Xavier, K., A. KC, N. A. Peres, Z. Deng, W. Castle, W. Lovett, and G. E. Vallad. 2019. “Characterization of Colletotrichum Species Causing Anthracnose of Pomegranate in the Southeastern United States.” Plant Disease 103 (11): 2771–2780. https://doi.org/10.1094/PDIS-03-19-0598-RE
Xavier, K. V., and G. E. Vallad. 2020. “Efficacy of Biological and Conventional Fungicide Programs for Foliar Disease Management on Pomegranate (Punica granatum) in Florida.” Plant Health Progress 21 (3): 199–204. https://doi.org/10.1094/PHP-02-20-0012-RS
Yu, X., K.V. Xavier, G. E. Vallad, and Z. Deng. 2018. “Disease Resistance in Pomegranates: Importance, Sources, Breeding Approaches, and Progress.” Proceedings of the Florida State Horticulture Society 131: 1–5. https://journals.flvc.org/fshs/article/view/114705/110032