Introduction
Production of fish in the US includes ornamental, food, bait, and game fish species. The value of aquacultured finfish in the US in 2023 by commodity was as follows: food fish, $820M; ornamental, $77.1M; sport fish, $54.4M; and baitfish, $48.1M (USDA 2023 Census of Aquaculture NASS). Florida aquaculture includes all of those commodity groups, with ornamental fish in Florida accounting for approximately 80–90 percent of all aquarium fish raised in the United States.
Aquaculture farmers breed and raise hundreds of species of finfish, using a variety of techniques. Some species will spawn after simple environmental changes have been made, such as changes in water temperature, pH, or conductivity. Other species require more advanced methods, including administration of hormone products by injection ("hormone-induced spawning").
One of these products, Ovaprim® (Syndel, Ferndale, WA), has been used worldwide for over a decade as a spawning aid for many different species of fish. Ovaprim® was available in the United States for many years for commercial ornamental fish breeders only as an FDA Investigational New Animal Drug (INAD) administered through the University of Florida's Tropical Aquaculture Laboratory. In March of 2009, Ovaprim® became the first new animal drug added to the FDA Index of Legally Marketed Unapproved New Animal Drugs for Minor Species (i.e., the Index) for use as a spawning aid in ornamental finfish broodstock. More recently, in June of 2025, the Ovaprim® FDA Index label was modified to allow use in all finfish broodstock, including ornamental fish, food fish, baitfish, and game fish broodstock which will not be consumed by humans or food-producing animals. Under the Index, Ovaprim® is intended for use as a spawning aid in finfish broodstock. More information on Ovaprim® can be found at the FDA Index website (https://www.fda.gov/animal-veterinary/minor-useminor-species/index-legally-marketed-unapproved-new-animal-drugs-minor-species). The first column lists drugs by MIF (Minor Species Index File) number, so look for MIF No. 900-001; Freedom of Information summaries and labeling information are found on the far right of this row.
An understanding of Ovaprim's mode of action and considerations for its use are critical for successful use of this drug during finfish production.
What Is "the index?"
The Minor Use Minor Species Animal Health Act of 2004 provides additional means by which veterinary drugs can be made legally available for diseases that would not otherwise be economically viable for manufacturers. One of these methods, development of the "Legally Marketed Unapproved New Animal Drug Index for Minor Species," better known as "The Index," uses a new, innovative process to legalize drugs for use in non-food minor species of animals (any species that is not one of the major species: cattle, horses, swine, chickens, turkeys, dogs, and cats) and non-food life stages of food-producing minor species. Minor species include all fish, reptiles, amphibians, and most exotic pets, zoo animals, and wildlife, in addition to some other cultured food animals (e.g., sheep, goats, bees).
In order for a drug to be added to the FDA's Index, the drug company's representative (the "requestor") must follow a series of steps. First, a request must be made to FDA-CVM to determine if the drug is eligible for addition to the Index. Next, a qualified expert panel, composed of a minimum of three persons with the training and experience to evaluate the drug, must be selected by the sponsor and accepted by FDA. Once accepted, the panel must thoroughly review all relevant documents and all other information (including experience with the drug) with regard to drug effectiveness and safety, ultimately producing a summary report with their findings and recommendations. After this has been submitted by the requestor to FDA, the FDA will review the report. If the panel's findings are in favor of addition of the drug to the Index, and FDA is in agreement, the drug will become a legally marketed, unapproved drug (USFDA Drug Indexing).
How is spawning triggered naturally?
Spawning, the release of gametes (mature oocytes/eggs by the female or of sperm/milt by the male) from the gonads (ovaries or testes), is the final event in the reproductive cycle (Rottmann et al. 1991a) and is the result of complex interactions between a variety of hormones and different tissues/organs in the fish's body.
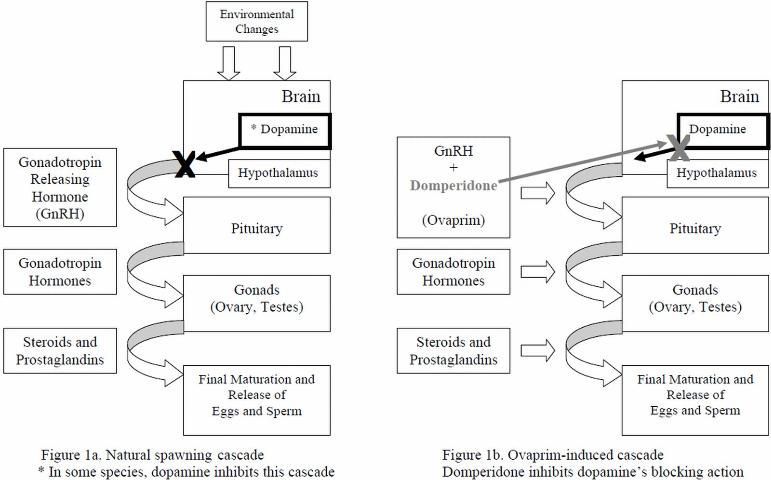
The following description is a very simplified version of major events (Rottmann et al. 1991a; Figure 1a) that occur during natural spawning. Environmental and internal factors (which differ from species to species) trigger a hormone cascade leading to egg maturation and release in females, and sperm maturation and release in males. First, a hormone called "gonadotropin-releasing hormone" (GnRH) is released from the hypothalamus portion of the brain and travels to the pituitary gland, which then releases gonadotropin hormones (especially GtH-II). The gonadotropin hormones/GtH-II travel to the ovaries and testes and stimulate them to produce steroids and prostaglandins, hormones that act directly on the gonads to cause final maturation and release of oocytes ("eggs") in females (ovulation), and the release of sperm in males (spermiation) (Rottmann et al. 1991a; Evans and Claiborne 2006). In some species of fish, a compound called dopamine naturally blocks this hormone cascade and inhibits egg and sperm production under certain conditions, for example, exposure to major stressors.
Credit:
What is Ovaprim®?
Ovaprim® is a two-part drug consisting of 1) a shorter, synthetic version of GnRH and 2) domperidone. Ovaprim® is marketed ready to inject in a liquid form.
In the ornamental fish industry, Ovaprim® is used as a spawning aid to induce ovulation (release of mature oocytes/eggs) and spermiation (release of milt/sperm) in mature, properly conditioned brood-fish. Ovaprim® is especially useful for species for which natural spawning in captivity is difficult to induce.
NOTE: Use of Ovaprim® does not guarantee successful fertilization, development, and hatch of fry. Overall good husbandry and hatchery management, including good genetics, proper nutrition, environment (including water quality), substrates, social structure, and other factors will affect egg and milt quality, egg hatch, and fry survival.
How does Ovaprim® work?
For some finfish species, determining which natural environmental cues will lead to ovulation and spermiation has been difficult. Ovaprim's synthetic GnRH closely resembles that of naturally occurring GnRHs and, in many species, is actually more potent (Figure 1b). When injected into the body cavity (coelom or “abdomen”) or muscle of receptive, mature, conditioned fish, the synthetic GnRH travels from the injection site through the blood to activation sites in the pituitary gland. Ovaprim® initiates the reproductive cascade and eliminates the need for a natural trigger. Domperidone, the other active component of Ovaprim®, helps block the inhibitory effects of dopamine. Domperidone, therefore, is very important for induced spawning of species for which the reproductive cascade would be stopped because of stressors that lead to dopamine release, because dopamine will block GnRH activity.
What do I need to know before I use it?
Ovaprim is intended for use as a spawning aid in reproductively mature, conditioned fish. The aquaculturist should know optimal water quality for both conditioning and spawning, including temperature; the approximate size and/or age of maturity in both males and females for a given species; appearance of mature, conditioned males and females; and methods for assessing gonadal development (for both ovaries and testes). Conditioning diet, although difficult to optimize for some species due to uncertainties, should be as complete and abundant as possible.
How do I check my fish for maturity?
For many fish species, sex determination is based on the differences in coloration, finnage, size, external structures (e.g., nuptial tubercles or “bumps” on many male cyprinid species or nuchal humps on the heads of many male cichlids), or body shape. In species where males and females look similar, one crude method that is often useful to determine sex of the fish is based on the larger volume of the eggs in conditioned females vs. sperm in males. Females may become more robust around the coelomic cavity (from enlarged ovaries due to egg yolk accumulation in late stages). In some cases, more robust fish may in fact be fat males. Consequently, careful collection and examination of eggs from the ovary (or sperm from the testes) is highly recommended for a more accurate assessment.
Catheterization and surgical biopsy are two different accurate methods that can be used to determine sex of the fish and maturity of the eggs (or sperm). For catheterization, a small-bore rubber tube (i.e., catheter) is placed into the genital pore/vent/opening and is rotated gently through the oviduct into the ovary. For koi, ~1.57 mm inner diameter (ID) /~2.41 mm outer diameter (OD) Silastic or other silicon tubing can be used. Once the tube is properly inserted, a sample of eggs may be extracted by gentle suction or by removing the catheter while blocking the open end. The eggs can then be "staged" (their developmental status determined) using a microscope (Rottmann et al. 1991c). For some species of fish, a more complicated, surgical approach via incision into the body cavity is necessary; however, this should be performed only by a qualified individual. Eggs that are in a late stage of development are uniform in size and large (yolk-filled). They have a germinal vesicle (nucleus) that has migrated away from the center of the egg to facilitate sperm entry (Rottmann et al. 1991c). Mature, conditioned males will often release milt during handling or after gentle pressure is applied to their coelomic cavity.
Ovaprim® should only be used once broodstock are determined to be properly conditioned and sexually mature with late-stage gonads.
What supplies do I need to use Ovaprim®?
- Tank(s) with aerated water for sedation
- Tank(s) with aerated water for recovery
- Sedative
- Syringes with permanently attached needles or with luer lock tips and separate needles (22–28 gauge)
- For smaller fish (100 grams or less), use of microliter (μL) syringes with smaller gradations (typically 100 μL volume divided into 1 μL increments) and with screw-on needles will make administration simpler.
What else should I know before I use Ovaprim®?
Because Ovaprim® is a viscous liquid, the use of a syringe with a luer lock tip or permanently attached needle is recommended. Slip-on type needles may eject from the pressure, so if you use them, be sure they are seated on the syringe tightly.
To avoid the introduction of bacteria or other pathogens via injection, use only sterile needles and syringes to withdraw Ovaprim® from the bottle, and to inject the fish. After withdrawing the correct amount required for the specific fish, carefully point the syringe and needle upward and depress the syringe to remove any excess air.
How do I determine the right dose?
For many species, a single dose at 0.5 mL Ovaprim® per kilogram fish body weight, which is equal to 0.5 μL/gram body weight, is required. There are 1000 μL per 1 mL. To calculate the volume of Ovaprim® needed, determine the weight of your fish and use the appropriate formula below:
(Weight of fish in kg) x 0.5 mL/kg = mL of Ovaprim® required.
(Weight of fish in lbs) x (kg/2.2 lbs) x 0.5 mL/kg = mL of Ovaprim® required.
(Weight of fish in grams) x 0.5 μL/gram = μL of Ovaprim® required.
For example, if your brood fish weighs 70 grams, then:
70 grams x 0.5 μL/gram = 35 μL of Ovaprim®
Additionally, species and environmental differences may warrant a slightly different regimen. Although a single dose works well for many species, a split dose may improve success. Producers may want to consider a split dose if—assuming all other considerations have been examined—repeated attempts of a single dose are unsuccessful. In this two-dose scheme, for warm water species, the first (or "priming") dose is administered using 10% of the total volume; the second dose is administered at least 6 hrs later using the remaining 90% volume.
Where do I inject the fish?
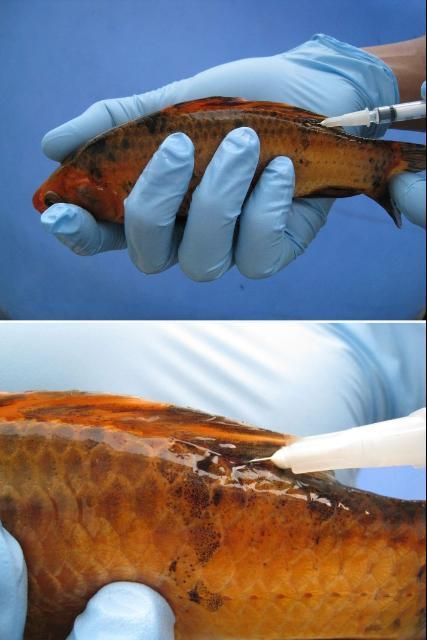
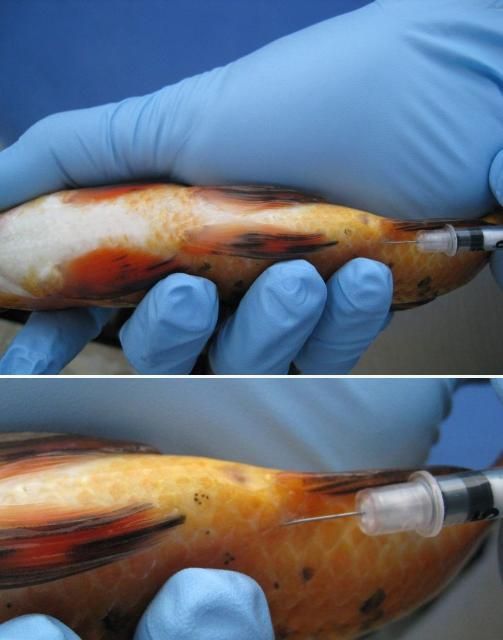
First, immobilize the fish using the correct dose of an anesthetic (e.g., pH buffered tricaine methanesulfonate ["MS-222,” Syncaine, Syndel USA, Ferndal, WA] or metomidate). Fish should be injected either in the muscle (IM) or in the body cavity (IP or ICe; i.e., in the "peritoneal cavity" or coelomic cavity) (see Figures 2 and 3). If you are injecting into the muscle (IM), a good area in many species is the dorsal (top) area of the fish, immediately behind or near the dorsal fin. If you follow a two-injection protocol, site the two injections on opposite sides of the fish. If you are injecting the fish into the body cavity (IP or ICe), turn it so that its ventral surface (belly) is up and its posterior (back ) end is slightly elevated. Inject into the body cavity in an area that is forward of but slightly off center to the anus or vent and be sure not to go too deep. If you make the injection too deep, you may accidentally inject into internal organs rather than into the body cavity itself. This positioning will help minimize the likelihood of injection into and damage to the liver, intestine, or other organs.
Credit:
Credit:
How will I know when the fish are ready to release their eggs or milt?
The length of time after the Ovaprim® injection when final maturation, ovulation, and spermiation will occur depends upon the species, the water temperature, and other factors. For some warm-water species, these events may occur as soon as 4 hours after the final injection, with others taking up to 12 hours. Consequently, fish should be inspected periodically after treatment. However, because time to ovulation or spermiation may vary, handling and disturbance should be minimized. Signs include a swelling or "softening" of the abdomen (coelom); presence of eggs in the water; easy expression of eggs or milt from broodstock with gentle pressure; or evidence of spawning behavior with mixed sex populations. In some groups, males may respond more rapidly than females.
Should I "strip" the fish or let them spawn naturally?
The decision whether to express the gametes (eggs and sperm) manually from the fish (i.e., to "strip" the fish) or to allow the fish to spawn on their own will depend upon several factors, including the species biology, number of males and females, availability of facility space and equipment, and the production scheme.
For some species and for some production schemes, stripping all males and females is preferred to minimize space and equipment requirements and to maximize production efficiency and synchronization of spawning. Manually stripping fish will often increase fertilization rates, allow for more uniform dispersal of fertilized eggs among production units, and prevent the brood fish from eating the eggs.
However, in some situations, manual stripping is not necessary or desirable. For some species, manual stripping is contraindicated because of biological considerations, including susceptibility to injury from handling, size, need for direct parental involvement, or other factors. In these situations, if there is ample system space, equipment, including proper spawning substrate, time, and adequate sex ratios, or if spawning is not well understood for a given species, aquaculturists may choose to allow the fish to spawn naturally.
If you are not familiar with how to manually strip fish for spawning, see Rottmann et al. (1991f).
For what species has the use of Ovaprim® been successful?
Ovaprim® has been used successfully in many different families and species of ornamental fish, including members of the family Cyprinidae (koi, goldfish, barbs, freshwater sharks), Characidae (pacu), Cobitiidae (loaches), different species of catfish (Order Siluriformes), and Helostomatidae (kissing gourami), in addition to other fish families and species. Ovaprim® has also been successfully used in various food, bait, and gamefish species, including members of the family Clariidae (African catfish and North African catfish), Cyprinidae (golden mahseer, grass carp, pengba, rohu, silver carp, shirbot, and snow trout), Percidae (Eurasian perch), Esocidae (northern pike), Haemulidae (pigfish), Serrasalmidae (black pacu), and Sparidae (black sea bream and pinfish) (see FDA Index Ovaprim links (MIF No. 900-001) located on the following site: https://www.fda.gov/animal-veterinary/minor-useminor-species/index-legally-marketed-unapproved-new-animal-drugs-minor-species).
What are some cases in which the use of Ovaprim® may not lead to the release of eggs or milt?
If you take into account all the considerations described in the sections above and follow the instructions, the chances of successful release of eggs and milt are greatly enhanced. However, there are a number of factors that may a) prevent Ovaprim® from working in a given situation; b) prevent successful stripping or spawning; or c) lead to complications (including disease or mortality) post-treatment. Some of these are listed below.
Fish-related considerations:
- Fish are not sexually mature and/or are not properly conditioned
- Fish have a subclinical disease (bacterial, parasitic, viral infection)
- Species may use a different form of GnRH
- Species may have a different initiating reproductive cascade and/or other inhibitors besides dopamine
- Females may have blockage in the oviduct, preventing expression of eggs
- Males testes may not have proper anatomy to permit manual stripping
Environment-related considerations:
- Incorrect water temperature
- Low dissolved oxygen
- High ammonia
- High nitrite
- Improper pH
- Improper conductivity or salinity
- Improper hardness or alkalinity
- Improper photoperiod
Procedure-related considerations:
- Incorrect dosage
- Fish handling is excessive or rough
- Injection made in the wrong site, or in a less than optimal site
- Ovaprim® has not been stored properly or is used after expiration date
- Non-sterile needles or syringes have been used, leading to infection
How should I store Ovaprim®?
Store below 77°F (25°C) and protect from sunlight and sources of heat.
How do I dispose of any unused product?
Contact your State Environmental Control Agency, or the Hazardous Waste Representative at the nearest EPA Regional Office for guidance pertaining to disposal of unused product.
Are there any human health or safety concerns with use of Ovaprim®?
As with all over-the-counter drugs, Ovaprim® poses minimal risk and is safe and effective when used as directed on the label. Use in a well-ventilated area. Wear gloves, goggles, and suitable protective clothing.
Ovaprim® is not intended for use in humans. Keep out of the reach of children. Avoid accidental contact (such as through inhalation, ingestion, or eye or skin contact) or self-injection. Seek medical advice immediately if you have concerns about inhalation, ingestion, eye or skin contact, or self-injection.
If you have any questions or problems concerning the use of Ovaprim®, contact the manufacturer and/or a knowledgeable production specialist immediately.
Summary
For some species of finfish, successful reproduction requires the use of hormone products. Ovaprim® is one such drug and consists of a synthetic GnRH and domperidone in a liquid propylene glycol carrier. Ovaprim® was the first new animal drug added to the FDA Index and can now be used as an over-the-counter injectable spawning aid in broodstock finfish.
Ovaprim® acts by stimulating a hormone cascade that results, ultimately, in the release of eggs and sperm. Ovaprim® will work successfully only in fish that are sexually mature, properly conditioned, and in the final stage of maturation.
Proper use of Ovaprim® will help facilitate synchronized and more efficient spawning of finfish species, including those for which the natural environmental cues needed for successful spawning have not been determined.
References and Further Reading
Abbas, G., R. Kasprzak, A. Malik, A. Ghaffar, A. Fatima, M. Hafeez-ur-Rehman, R. Kausar, S. Ayub, and N. Shuaib. 2019. Optimized spawning induction of blackfin sea bream, Acanthopagrus berda (Forsskal, 1775) in seawater ponds using Ovaprim® hormone, with general remarks about embryonic and larval development. Aquaculture 512:734387.
Abubakar M.Y., and J.K. Ipinjolu. 2019. Spawning Performance of Heterobranchus bidorsalis in Sokoto Dry Sub Humid Nigeria Using Ovaprim® C and Ovatide Hormones. Asian Journal of Emerging Research 1(4): 141–153.
Achionye-Nzeh, C.G., and I.S.R.A.E.L. Obaroh. 2012. Ovaprim® doses effects on eggs of African mudfish Clarias gariepinus. International Journal of Life Science and Pharma Research 2(2): 6–9.
Ali, M.A., S.B. Rasheed, Z.H. Zaigham-Hassan, M.I. Muhammad Ibrar, A.M. Abdul Majeed, Z.U. Zafar Ulhaq, H.J. Hamdullah Jan, Y.J. Yahya Jan, A.H. Abul Hasanat, M.S. Qureshi, and H.K. Hamid Khan. 2015. Efficacy of synthetic Freedom of Information Summary MIF 900-001 6 hormones Ovatide and Ovaprim® in induced breeding of major Indian and Chinese carps. Journal of Agricultural Technology 11(7): 1449–1456.
Ameer, M.W., F. Jabeen, M. Asad, G. Kaukab, A. Bashir, M. Rasheed, H. Younis, N. Munir, J. Nawaz, R. Zainab, and M. Akram. 2021. Comparative efficacy of Ovaprim® and hMG (menotropin) to induce breeding in African catfish (Clarias gariepinus). Fish Physiology and Biochemistry 47(5): 1559–1564.
Angel, J.R.J., V.K. Tiwari, P.S. Babu, K.D. Rawat, B. Ignatius, R.P. Kiran, S. Roy, R. Charan, D.R. Nair, P.S. Rao, and K.B. Sreeramamurty. 2015. Captive breeding of a near threatened fish, pengba Osteobrama belangeri (Valenciennes, 1844) using three different inducing agents. Indian Journal of Fisheries 62(4): 66–70.
Broach, J.S., C.L. Ohs, M.A. DiMaggio, C.C. Green, and D.R. Yante. 2015. Efficacy of varying doses of pituitary extract from channel catfish, Ictalurus punctatus, on spawning performance of pinfish, Lagodon rhomboides, and pigfish, Orthopristis chrysoptera. North American Journal of Aquaculture 77: 245–254.
Cheah, M.S.H., and C.L. Lee. 2000. Induced ovulation of the Australian eel-tailed catfish Neosiluris ater (Perugia) with Ovaprim. Asian Fisheries Science 13:87–96.
Evans, D.H., and J.B. Claiborne. 2006. The physiology of fishes. Third edition. Taylor and Francis, CRC Press, 601 pp.
Haniffa, M.A., P.S. Allen Benziger, A.J. Arockiaraj, M. Nagarajan, and P. Siby. 2007. Breeding behaviour and embryonic development of koi carp (Cyprinus carpio). Taiwania 52(1): 93–99.
Hill, J.E., J.D. Baldwin, J.S. Graves, R. Leonard, J.F.F. Powell, and C.A. Watson. 2005. Preliminary observations of topical gill application of reproductive hormones for induced spawning of a tropical ornamental fish. North American Journal of Aquaculture 67:7–9.
Hill, J.E., K.H. Kilgore, D.B. Pouder, J.F.F. Powell, C.A. Watson, and R.P.E. Yanong. 2009. Survey of Ovaprim use as a spawning aid in ornamental fishes in the United States as administered through the University of Florida Tropical Aquaculture Laboratory (2005–2007). North American Journal of Aquaculture 71(3): 206–209.
Ovaprim Freedom of Information Summary. https://www.fda.gov/animal-veterinary/minor-useminor-species/foi-summary-ovaprim (Accessed July 14, 2025)
Powell, J.F.F., J. Brackett, and J.A. Battaglia. 1998. Induced and synchronized spawning of captive broodstock using Ovaplant and Ovaprim. Bulletin of the Aquaculture Association of Canada 3:14–18.
Powell, J.F.F., S.L. Krueckl, P.M. Collins, and N.M. Sherwood. 1996. Molecular forms of GnRH in three model fishes: rockfish, medaka and zebrafish. Journal of Endocrinology 150:17–23.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991a. SRAC 0421 Introduction to Hormone-Induced Spawning of Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991b.SRAC 0422 Capturing, Handling, Transporting, Injecting and Holding Brood Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991c. SRAC 0423 Determining Sexual Maturity of Broodstock for Induced Spawning of Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991d. SRAC 0424 Hormonal Control of Reproduction in Fish for Induced Spawning. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991e. SRAC 0425 Hormone Preparation, Dosage Calculation, and Injection Techniques. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991f. SRAC 0426 Techniques for Taking and Fertilizing the Spawn of Fish. Southern Regional Aquaculture Center. USDA.
Rottmann, R.W., J.V. Shireman, and F.A. Chapman. 1991g. SRAC 0427 Induction and Verification of Triploidy in Fish. Southern Regional Aquaculture Center. USDA.
Sahoo, S.K., S.S. Giri, and A.K. Sahu. 2005. Induced spawning of Asian catfish, Clarias batrachus (Linn.): effect of various latency periods and sGnRHa and domperidone doses on spawning performance and egg quality. Aquaculture Research 36:1273–1278.
Sarkar, U.K., P.K. Deepak, R.S. Negi, S. Singh, and D. Kapoor. 2006. Captive breeding of endangered fish Chitala chitala (Hamilton-Buchanan) for species conservation and sustainable utilization. Biodiversity and Conservation. 15:3579–3589.
Syndel (Ferndale, WA). Ovaprim. https://syndel.com/product/ovaprim/ (Accessed July 14, 2025)
USDA 2023 Census of Aquaculture. National Agricultural Statistics Service. https://www.nass.usda.gov/Publications/AgCensus/2022/Online_Resources/Aquaculture/index.php (Accessed July 14, 2025)
US FDA Drug Indexing. https://www.fda.gov/animal-veterinary/minor-useminor-species/drug-indexing (Accessed July 14, 2025)
US FDA MUMS Index List. https://www.fda.gov/animal-veterinary/minor-useminor-species/index-legally-marketed-unapproved-new-animal-drugs-minor-species (Accessed July 14, 2025)