This Ask IFAS document is the third in the Seafood Guide series and provides information to the seafood industry on how to develop a seafood HACCP plan effectively.
Title 21 Code of Federal Regulation – Part 123 includes seafood Hazard Analysis Critical Control Point (HACCP) regulation, which requires processors and importers of fish and fishery products to develop and implement a HACCP plan to maintain the safety of their products and prevent foodborne outbreak (FDA 2023). The United States Food and Drug Administration (FDA)’s Fish and Fishery Products Hazards and Controls guidance (Figure 1) is a comprehensive document that outlines the FDA’s current recommendations for controlling seafood’s process- and species-related hazards (FDA 2022). The FDA’s guidance is an instrumental tool for developing a seafood HACCP plan. This Ask IFAS publication provides an overview of the guidance’s format and how to use it effectively to develop a seafood HACCP plan. The PDF version of the guidance is available for free at Florida Sea Grant and FDA’s websites. The printed version is available for purchase at the UF/IFAS Bookstore. The guidance also allows seafood processors and importers to use alternative approaches if the approaches meet the requirements of the applicable statutes and regulations.

Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
The Format of the FDA’s Guidance
The FDA’s guidance is updated intermittently to make sure its content reflects any updates in the regulation and science. The processors are responsible for updating their HACCP plan if any update in the guidance affects the hazards associated with their fish species and process. Page number style can help readers to distinguish updated contents from original contents in FDA’s guidance. Figure 2 has provided examples of the page numbering formats. The chapters that have the original content show a “traditional” style of numbering (e.g., Chapter 4, p. 75) whereas chapters that have been updated have “– style” numbering (e.g., Chapter 9, p. 9 – 1).

Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
The FDA’s guidance document includes 21 chapters, 12 appendices, and 2 addendums. Table 1 includes the chapters, appendices, addendums, and their associated page number in the guidance document.
Table 1. FDA’s Fish and Fishery Products Hazards and Controls guidance sections and page numbers since last updated in June 2022.
Notes on Chapter 3
The FDA’s guidance has systematically divided the potential biological, chemical, and physical hazards into species- and process-related hazards in Chapter 3. Chapter 3 includes three tables for identifying species- and process-related hazards as follows:
- Table 3-2, “Potential Vertebrate Species-Related Hazards,” contains a list of potential hazards that are associated with specific species of vertebrates (species with backbones).
- Table 3-3, “Potential Invertebrate Species-Related Hazards,” contains a list of potential hazards that are associated with specific species of invertebrates (species without backbones).
- Table 3-4, “Potential Process-Related Hazards,” contains a list of potential hazards that are associated with specific finished fishery products, as a result of the finished product form, the package type, and the method of distribution and storage.
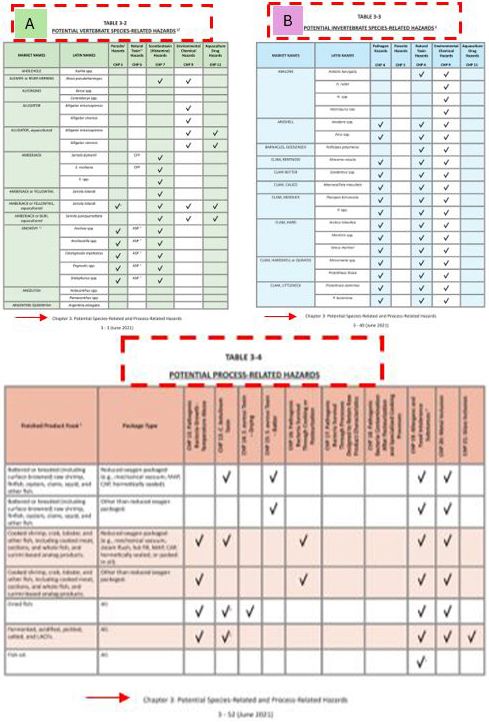
Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
These tables are in alphabetical order with the market names of the species. Seafood processors can also identify the market name of their products using the “FDA Seafood List” (FDA 2022).
Notes on Chapters 4 to 21
Chapters 4 to 21 of the FDA’s guidance contain the information for various species- and process- related hazards. These chapters have the exact same format, and understanding the format is imperative for effective use of the guidance document. The sections of chapters of the guidance document are illustrated below, using Chapter 16, “Pathogenic Bacteria Survival Through Cooking or Pasteurization,” as an example.
- UNDERSTAND THE POTENTIAL HAZARD: This section includes background information about the hazard.

Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
2. DETERMINE WHETHER THE POTENTIAL HAZARD IS SIGNIFICANT: Using this section, you can identify whether the potential hazard is significant and reasonably likely to happen.
3. IDENTIFY CRITICAL CONTROL POINTS: Use this section to identify if the potential hazard should be controlled at a specific step in the processing or not. Any step of the processing where the hazards should be controlled to prevent foodborne outbreak will be considered a CCP.

Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
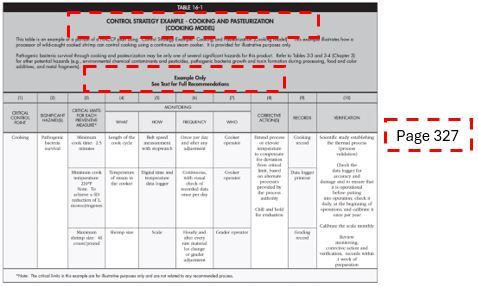
4. DEVELOP A CONTROL STRATEGY: Using this section in each chapter, you can set critical limits for the species- or process-related hazard and establish your monitoring procedures, corrective actions, record-keeping system, and verification procedures.

Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
Additionally, each chapter has an example of a portion of a HACCP plan for a specific control strategy that is provided at the end of chapter. These examples can be used as resources for developing the HACCP plan. However, the FDA’s guidance text must be used to understand the full recommendation for developing the HACCP plan.
Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
How to Use the FDA’s Guidance to Develop a HACCP Plan
The FDA’s guidance document provides a series of 18 steps to seafood processors to conduct the hazard analysis and develop a HACCP plan. The corresponding Hazard Analysis Worksheet and HACCP plan form are developed by the Seafood HACCP Alliance and can be downloaded from Florida Sea Grant’s website. However, there are no standardized forms, and seafood processors can use other forms as long as they have the required components for developing a HACCP plan.
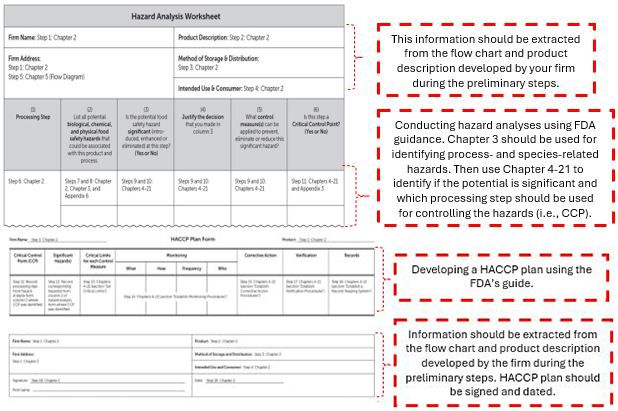
Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
Additional Information
- When using tables in Chapter 3, pay attention to the superscripts in the table. These can affect your hazard identification. At the end of each table in Chapter 3, you can find the detailed description of each superscript. The example in Figure 9 shows the importance of these superscripts for hazard identification.
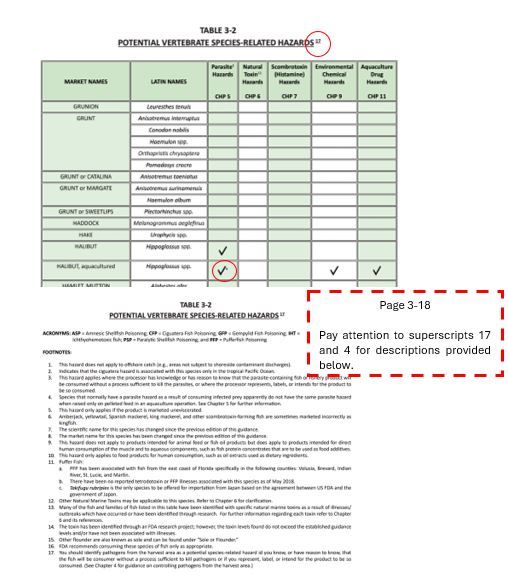
Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
2. When using Table 3-4 of the FDA’s guidance for identifying the process-related hazards, pay attention to the packaging types because they can affect the process-related hazard identification.

Credit: FDA’s Fish and Fishery Products Hazards and Controls guidance
3. When developing a seafood HACCP plan, conduct a hazard identification for any other ingredients and processes that are involved in the production of fisheries products (e.g., rice acidification in sushi preparation, or ingredients such as egg and wheat for bread and battering the products).
Conclusion
The FDA’s Fish and Fishery Products Hazards and Controls guidance is a resource that includes the agency’s current thinking and recommendations for fish and fishery products processors and importers to develop and implement a HACCP plan. The guidance document can be used to identify the process- and species-related biological, chemical, and physical hazards, identify the CCP, and develop the seafood HACCP plan.
References
FDA. 2023. “CFR—Code of Federal Regulations Title 21.” https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=120.6
FDA. 2022. Fish and Fishery Products Hazards and Controls. Fourth edition. https://www.fda.gov/food/seafood-guidance-documents-regulatory-information/fish-and-fishery-products-hazards-and-controls
FDA. 2022. “Guidance for Industry: The Seafood List FDA’s Guide to Determine Acceptable Seafood Names.” https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-seafood-list-fdas-guide-determine-acceptable-seafood-names