Introduction
Flies of the genus Pseudacteon are members of the family Phoridae, commonly known as scuttle flies, hump-backed flies, and phorid flies (Disney 1994). Phorid flies in the genus Pseudacteon parasitize several genera of ants including fire ants in the genus Solenopsis (Disney 1994), which includes the invasive red (Solenopsis invicta Buren) and black imported fire ants (Solenopsis richteri Forel). In 1997, Pseudacteon tricuspis Borgmeier was the first species of Pseudacteon fly successfully released as a biological control agent for imported fire ants in the US (Callcott et al. 2011). Five other species, Pseudacteon curvatus Borgmeier, Pseudacteon litoralis Borgmeier, Pseudacteon obtusus Borgmeier, Pseudacteon nocens Borgmeier, and Pseudacteon cultellatus Borgmeier have since been released (Porter and Calcaterra 2013). Eight native Pseudacteon species parasitize native fire ants in the United States (Plowes 2009).

Credit: Sanford D. Porter, USDA-ARS.
Distribution
Pseudacteon flies are parasitoids of ants in many genera worldwide (Disney 1994). About two dozen species parasitize native fire ants in South America (Porter and Pesquero 2001). In the United States, the range of introduced species varies according to their rates of expansion and dates of introduction (Drees and Oi 2015). Pseudacteon triscuspis and Pseudacteon curvatus are distributed across most of the southeastern US (Callcott et al. 2011). Pseudacteon litoralis populations are occasionally abundant in Alabama (Porter et al. 2011), as are Pseudaceton nocens in Texas (Plowes et al. 2012). Both species currently have limited but expanding ranges. Pseudacteon obstusus is abundant in a large portion of both Texas and Florida, with smaller established populations in Mississippi and Georgia (Drees and Oi 2015, Plowes et al. 2011, Porter and Calcaterra 2013). Pseudacteon cultellatus is the most recently released species and is established at two sites in Florida (Porter et al. 2013).
Description
Eggs
Eggs are approximately 130 µm long by 20 µm wide and torpedo-shaped (Zacaro and Porter 2002).
Larvae
Early instars are maggot-like and transparent, becoming broader, flatter, and cloudy-whitish as they mature (Porter 1998).
Pupa
Early instars are maggot-like and transparent, becoming broader, flatter, and cloudy-whitish as they mature (Porter 1998).
Adults
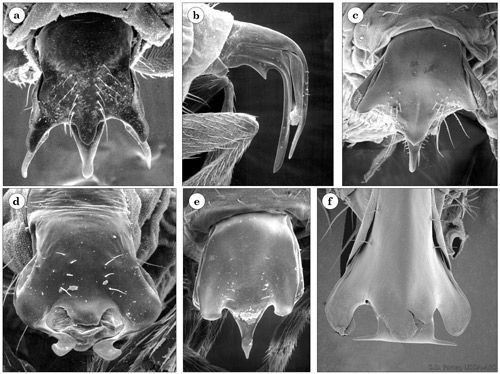
Phorid flies are small to very small with a hump-backed appearance and reduced wing venation (Figure 1) (Triplehorn and Johnson 2005). Female Pseudacteon flies have fully developed wings and unique and highly sclerotized external ovipositors (Porter and Pesquero 2001). The ovipositor, an organ used for egg-laying, is different in appearance for each species of Pseudacteon and can be used to distinguish one species from another (Figure 2). Males are sometimes smaller than females.
Credit: Sanford D. Porter, USDA-ARS
Biology
Pseudacteon flies have a unique life history. In fire ant attacking Pseudacteon flies, the mated female will hover over foraging workers (Figure 3) and strike an ant in the thorax with her ovipositor, depositing an egg into the ant's body (Porter 1998). These strikes are often very fast, occurring in less than a second. After the larva hatches, it moves into the ant's head and consumes mostly hemolymph until just prior to pupation after the third instar. When the larva is ready to pupate it releases a chemical, likely an enzyme, that degrades the membranes that hold the ant's exoskeleton together. The larva then consumes the contents of the ant's head, upon which the head usually falls free of the body (Figure 4). Through a series of repetitive body extensions, the larva forces the deceased ant's mouthparts off the head, though sometimes they do not completely detach (Figure 6). During pupation, the first three segments of the larva's body become sclerotized and fuse into a plate. This plate fills the oral cavity where the ant's mouth parts were previously attached. Within a couple of days, two respiratory structures extend from the sides of the puparium (Figure 5). The adult fly will emerge from the ant's head 2-6 weeks after pupation (Figure 6). The total development time is 4-12 weeks, depending on temperature, and time as an adult represents only about 3-5 days of this time (Pesquero et al. 1995, Porter et al. 1997, Porter 1998).
An interesting mechanism for sex determination exists in several species of Pseudacteon wherein sex is determined by the size of the head capsule in which pupation occurs. Large ants result in female flies, and small ants result in male flies (Morrison et al. 1999).

Credit: Sanford D. Porter, USDA-ARS

Credit: Sanford D. Porter, USDA-ARS

Credit: Sanford D. Porter, USDA-ARS

Credit: Sanford D. Porter, USDA-ARS
Hosts
Pseudacteon flies released as biological control agents in the United States primarily attack the red imported fire ant, Solenopsis invicta Buren, as it is the most prevalent introduced species (Callcott and Collins 1996). Pseudacteon litoralis and Pseudacteon curvatus also will attack black imported fire ants, Solenopsis richteri Buren, and Solenopsis invicta × Solenopsis richteri hybrids (Callcott et al. 2011, Porter et al. 2011) where they occur. Pseudacteon species that are native to North America will attack native fire ants and ants in other genera (Disney 1994; Plowes et al. 2009). All Pseudacteon species have been observed to be highly host specific, only attacking ants from a single species or a group of closely related species (Porter and Gilbert 2004, Weissflog et al. 2008). Whereas non-parasitic phorid flies often are found near decaying matter, and some species can become household pests, ant-decapitating Pseudacteon flies are not attracted to people or animals (other than ants), decaying matter, carrion, feces, fruits, vegetables, or prepared foods (Porter and Gilbert 2004). Pseudacteon flies will only be found around disturbed fire ant mounds.
Biological Control
Invasive imported fire ants have been present in the United States since around 1918 (Callcott and Collins 1996). In their native range of South America, fire ant populations are much lower than in the United States. Porter (1998) surmises that this difference in population density is due to the absence of natural enemies in their adventive range. In addition to the direct impacts of parasitism, the presence of Pseudacteon flies has been shown to interrupt fire ant foraging by triggering immediate defensive and evasive behaviors (Porter 1998). The ability of fire ants to recognize Pseudacteon flies and their strong reactions to the presence of the flies strengthened interest in use of Pseudacteon spp. for biological control. Around 1994, efforts to import Pseudacteon flies began with the initiation of two separate research programs; one by the USDA-ARS fire ant research unit in Gainesville, FL and the other at the University of Texas in Austin, TX (Callcott et al. 2011). The first successful release of a Pseudacteon fly occurred in 1997 with the release of Pseudacteon tricuspis in Florida. From 1997 on, USDA-ARS, the University of Texas, and USDA-APHIS in cooperation with 11 states have released six different species of Pseudacteon at numerous sites across the southern US. The biological control of invasive imported fire ants is an ongoing effort that also includes various other parasitoids and pathogens including microsporidians, viruses, and other parasites (Briano et al. 2012).
Selected References
Briano J, Calcaterra L, Varone L. 2012. Fire ants (Solenopsis spp.) and their natural enemies in southern South America. Psyche: A Journal of Entomology 2012: 19 pp.
Callcott AA, Collins HL. 1996. Invasion and range expansion of imported fire ants (Hymenoptera: Formicidae) in North America from 1918-1995. Florida Entomologist 79: 240-251.
Callcott AM, Porter SD, Weeks RD, Graham LC, Johnson SJ, Gilbert LE. 2011. Fire ant decapitating fly cooperative release programs (1994-2008): Two Pseudacteon species, P. tricuspis and P. curvatus, rapidly expand across imported fire ant populations in the southeastern United States. Journal of Insect Science 11: 19.
Disney RHL. 1994. Scuttle Flies: The Phoridae. Chapman & Hall, London. 467 pp.
Drees BM, Oi D. 2015. Natural Enemies of Fire Ants. eXtension.org. (31 May 2016)
Morrison LW, Porter SD, Gilbert LE. 1999. Sex ratio variation as a function of host size in Pseudacteon flies (Diptera: Phoridae), parasitoids of Solenopsis fire ants (Hymenoptera: Formicidae). Biological Journal of the Linnaean Society 66: 257-267.
Pesquero MA, Porter SD, Fowler HG, Campiolo S. 1995. Rearing of Pseudacteon spp. (Dipt., Phoridae), parasitoids of fire ants (Solenopsis spp.)(Hym., Formicidae). Journal of Applied Entomology 119: 677-678.
Plowes RM, Lebrun EG, Brown BV, Gilbert LE. 2009. A Review of Pseudacteon (Diptera: Phoridae) that parasitize ants of the Solenopsis geminata complex (Hymenoptera: Formicidae). Annals of the Entomological Society of America 102: 937-958.
Plowes RM, LeBrun EG, Gilbert, LE. 2011. Introduction of the fire ant decapitating fly Pseudacteon obtusus in the United States: Factors influencing establishment in Texas. BioControl 56: 295-304.
Plowes, RM, Folgarait PJ, Gilbert LE. 2012. The introduction of the fire ant parasitoid Pseudacteon nocens in North America: challenges when establishing small populations. BioControl 57: 503-514.
Porter SD, Williams DF, Patterson RS. 1997. Rearing the decapitating fly Pseudacteon tricuspis (Diptera: Phoridae) in imported fire ants (Hymenoptera: Formicidae) from the United States. Journal of Economic Entomology. 90: 135-138.
Porter SD. 1998. Biology and behavior of Pseudacteon decapitating flies (Diptera: Phoridae) that parasitize Solenopsis fire ants (Hymenoptera: Formicidae). Florida Entomologist 81: 292-309.
Porter SD, Calcaterra LA. 2013. Dispersal and competitive impacts of a third fire ant decapitating fly (Pseudacteon obtusus) established in North Central Florida. Biological Control 64: 66-74.
Porter SD, Gilbert LE. 2004. Assessing host specificity and field release potential of fire ant decapitating flies (Phoridae: Pseudacteon). pp. 152-176, In Van Driesche RG, Reardon R [eds.] Assessing host ranges for parasitoids and predators used for classical biological control: A guide to best practice. Forestry Health Technology Enterprise Team (FHTET). USDA Forest Service, Morgantown, West Virginia.
Porter SD, Graham LC, Johnson SJ, Thead LG, Briano JA. 2011. The large decapitating fly Pseudacteon litoralis (Diptera: Phoridae): Successfully established on fire ant populations in Alabama. Florida Entomologist 94: 208-213.
Porter SD, Kumar V, Calcaterra LA, Briano JA, Seal DR. 2013. Release and establishment of the little decapitating fly Pseudacteon cultellatus (Diptera: Phoridae) on imported fire ants (Hymenoptera: Formicidae) in Florida. Florida Entomologist 96: 1567-1573.
Porter SD, Pesquero MA. 2001. Illustrated key to Pseudacteon decapitating flies (Diptera: Phoridae) that attack Solenopsis saevissima complex fire ants in South America. Florida Entomologist 84: 691-699.
Triplehorn CA, Johnson NF. 2005. Borror and DeLong's Introduction to the Study of Insects, seventh edition.
Weissflog A, Maschwitz U, Seebauer S, Disney RHL, Seifert B, Witte V. 2008. Studies on European ant decapitating flies (Diptera: Phoridae): II. Observations that contradict the reported catholicity of host choice by Pseudacteon formicarum. Sociobiology 51: 87-94.
Zacaro AA, Porter SD. 2003. Female reproductive system of the decapitating fly Pseudacteon wasmanni Schmitz (Diptera: Phoridae). Arthropod Structure and Development 31: 329-337.