Abstract
Root-knot nematodes (Meloidogyne spp.) are one of the main soilborne pathogens in Florida and cause considerable damage to many vegetables. Asian vegetables are important specialty crops in Florida, but no information is available on their host status to plant-parasitic nematodes, in particular to Meloidogyne spp. Therefore, we conducted a survey to learn which Asian vegetables are grown in Florida, the plant-parasitic nematodes associated with these crops, and the damage these nematodes could potentially cause to Asian vegetables.
Introduction
Because this publication is the first of its kind on plant-parasitic nematodes in Asian vegetables, it will likely raise awareness among growers and researchers in Florida and the United States about plant-parasitic nematodes in Asian vegetables. It also provides recommendations for managing plant-parasitic nematodes, especially root-knot nematodes (Meloidogyne spp.), the most important nematode threat in Florida’s vegetable production. This publication also serves as a resource for identification and management of nematodes for UF/IFAS Extension agents, researchers, and academics working on Asian vegetables.
Plant-parasitic nematodes are microscopic roundworms that feed on living plant tissues. Most nematode species feed on the belowground parts of plants like roots and tubers, although some species feed on aboveground plant parts, which can be seen on several ornamental crops and strawberry in Florida. There are no typical aboveground symptoms of nematodes feeding on roots, and often their damage may be misidentified with other causes such as nutrient or water deficiency or diseases related to bacteria or fungi (stunting, wilting, and yellowing). Because the damage plant-parasitic nematodes cause is often not recognized or is mistaken for other problems, they are often called the unseen enemy. Plant-parasitic nematodes are a costly burden for agricultural production with an estimated $125 billion loss annually (Bernard et al. 2017). In Florida, year-round warm weather and high humidity create a perfect habitat for many plant-parasitic nematodes, and nematode damage can be very severe, especially in sandy soils.
Asian Vegetable Production In Florida
The demand for ethnic Asian vegetables in the United States is rapidly increasing, and Asian vegetables have become one of the most popular specialty crops (Kaiser and Ernst 2018). The growing popularity of Asian restaurants and the demand of American consumers for Asian vegetables in their diet has created a rapid increase in the acreage of Asian vegetable production in the United States. In Florida, the year-round warm climate is very favorable for growing many Asian vegetables. Florida producers currently grow more than 40 Asian vegetables, with a rapid expansion of ethnic Asian vegetable production in northeast and south Florida. Growing ethnic Asian vegetables can bring many benefits to Florida such as creating more job opportunities, diversifying crops for crop rotation, and improving human health (given that many Asian vegetables contain various medicinal compounds) (Liu 2016). Currently, Asian vegetables are grown on more than 4,000 ha in Florida for local consumption and, especially in the winter, for export to northern states (New York, New Jersey, Massachusetts, Connecticut, Illinois, etc.) (Gu et al. 2021). Limited guidance for management of weeds, insects and diseases affecting some Asian vegetables has been published in the Vegetable Production Handbook of Florida (Vegetable Production Handbook of Florida 2022), but no information or recommendations are available for plant-parasitic nematode management on Asian vegetables.
Plant-Parasitic Nematodes in Asian Vegetables
Many of the Asian vegetable growers in Florida are of Vietnamese origin, and due to language and cultural barriers, they are often very isolated. Farm size can vary but farms are usually between 5 and 100 acres, with a few exceptions that are more than 1,000 acres. Most Asian vegetables on small and mid-size farms are grown in plastic tunnels on wooden frames that are closed during winter and are uncovered during the summer (Figure 1).

Credit: Hung X. Bui, UF/IFAS
To better understand and educate Asian vegetable growers about nematodes, a survey was done of Asian vegetable farms owned and operated by Vietnamese growers in Wimauma, Hillsborough County, Florida. The objective was to learn more about the crops that are grown and if the nematodes are a problem. Most farmers were unaware of a nematode problem, and, in most cases, also unaware of the existence of nematodes, but results indicated that all 10 surveyed farms showed visible nematode damage in the form of stunted and chlorotic plants (Figure 2) and galled roots caused by root-knot nematodes (Figure 3). Twenty different vegetables were sampled, mostly leafy, but also some fruiting and root or tuber vegetables (Table 1). Thai basil and water spinach were the most common vegetables and were found on all surveyed farms (Figure 4).

Credit: Hung X. Bui, UF/IFAS

Credit: Hung X. Bui and Mengyi Gu, UF/IFAS

Credit: Hung X. Bui, UF/IFAS
Several plant-parasitic nematodes were isolated and identified from the soil or roots collected from these surveyed farms, including root-knot, sting, stubby root, and lesion nematodes (Table 1). Root-knot nematodes were the most common and appeared to cause the most damage. Root gall size varied from small (Thai basil, ginger, jute) to large (sweet potato, water spinach, luffa, Vietnamese muskmelon, Vietnamese eggplant) (Figure 3). Thai basil, water spinach, pumpkin, Malabar spinach, Indian taro, sweet potato, jute, bitter gourd, luffa, and Vietnamese eggplant also showed visible aboveground symptoms, such as leaf yellowing and wilting. Four different root-knot nematode species (Meloidogyne spp.) were identified: Meloidogyne arenaria, M. enterolobii, M. haplanaria, and M. incognita. Among these four species, M. enterolobii accounted for 74% of the samples identified, M. arenaria and M. incognita were each found in 10% of the samples, and M. haplanaria was found in 6% of the samples (Bui and Desaeger 2022). Meloidogyne spp. are the most well-known and damaging plant-parasitic nematodes globally and are extremely common in Florida’s warm climate and sandy soils. Meloidogyne spp. are sedentary endoparasitic nematodes, which means they enter plant roots completely (the entire nematode is inside the root) and complete most developmental stages inside the roots (Figure 5). The nematode develops within an eggshell from an embryo through the first and second juvenile stages within an eggshell. The infective second-stage juveniles (J2s) hatch and enter the root elongation zone and then moves upward to the root tips where they invade the vascular cylinder to form a feeding site of three to ten cells, called a giant cell. Simultaneously, the neighboring cells start to divide to form a typical gall or root-knot. Male and female J3s become round and sedentary inside the gall and continuously molt to J4s and then adults. Adult females commonly reproduce asexually through parthenogenesis. In some Meloidogyne spp., they reproduce through sexual reproduction, in which adult males remodel back to a thread-like shape, while adult females keep their pear shape. Adult males fertilize females and leave the root afterward. The life cycle of Meloidogyne spp. can be completed in 3–4 weeks under ideal environmental conditions. A single female can lay 500 to 1000 eggs during its lifetime (Abad et al. 2008).
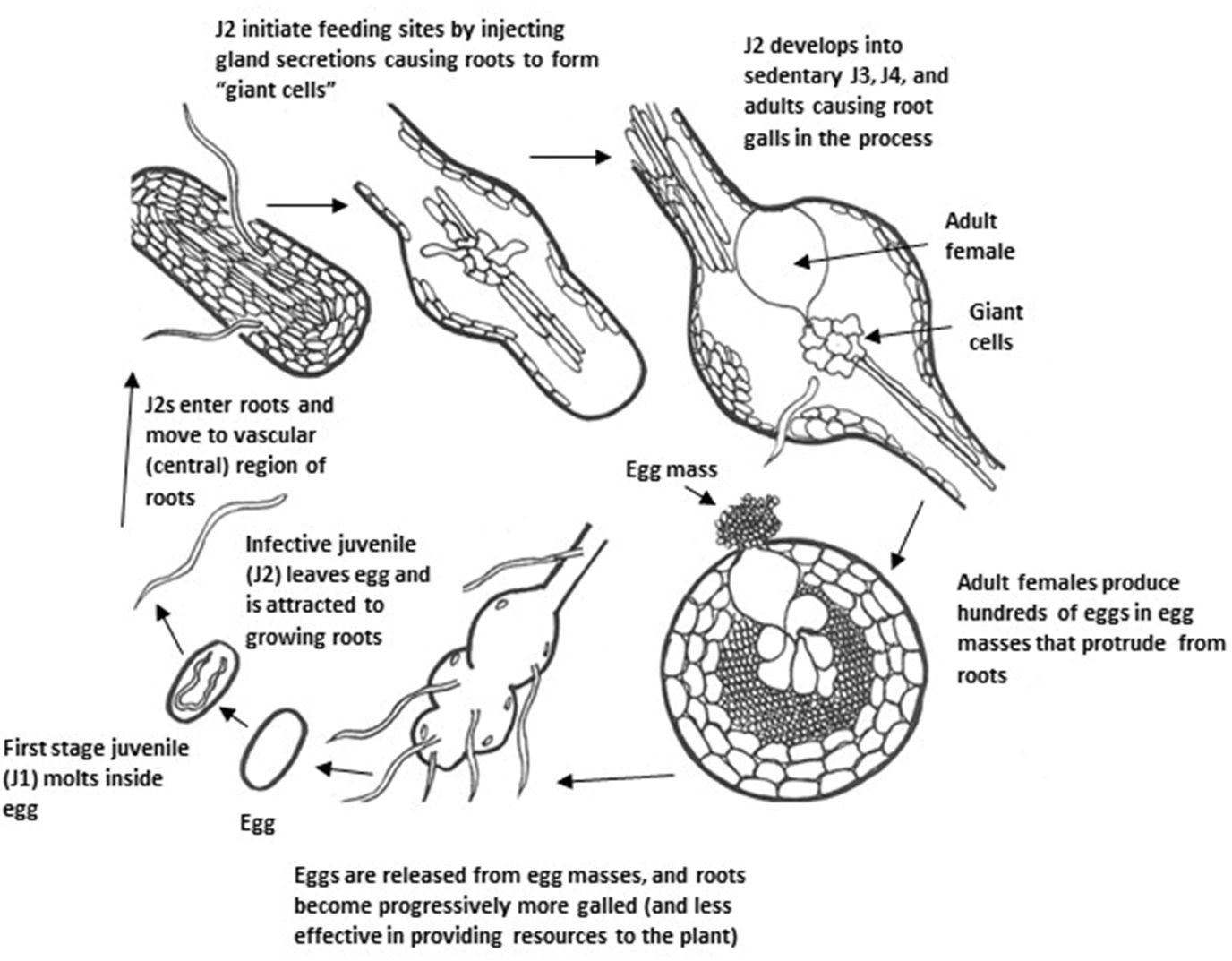
Credit: Courtesy H. Regier, adapted from G. Abawi and V. Brewster
Root-knot nematodes are one of the main reasons for the use of soil fumigants in much of Florida’s high-value crop production systems (Noling 1996). Many reports have confirmed the host status of ornamental plants, turfgrass, tomato, and other vegetables to various Meloidogyne spp. in Florida (Brito et al. 2007; 2008; 2010; Baidoo et al. 2016; Joseph et al. 2016; Crow 2020). The high prevalence of M. enterolobii in our survey is of concern. Many of the surveyed farms were established at least 10–30 years ago, and it is impossible to tell how long M. enterolobii has existed in these fields. Meloidogyne enterolobii may have been spread across these Asian vegetable farms because farmers were unaware that they were planting infested plant material. Asian farmers are frequently isolated by language barriers and may have little to no access to Extension guidelines and pest management information. None of the farmers that we visited had ever heard about plant-parasitic nematodes and the risk they could pose to the farmers’ crops. As a result, they were practicing no nematode management and had no information on losses that nematodes were causing on their farms. The diversity and continuous production of crops—sometimes more than 10 crops are grown on the same farm—further complicates management recommendations for these farmers. Rotation and cover crop options are limited, especially considering that most crops are good hosts to root-knot nematodes and that fields are being planted almost year-round.
Nematode Management
Chemical Control
Few chemicals are labeled for Asian vegetable crops, and most growers do not have a pesticide license due to the language barrier. Hence, chemical control products for nematode management are not recommended to these Asian vegetable growers. While some biological nematicides can be used for nematode management, their efficacy has not been demonstrated for Asian vegetables.
Non-Chemical Control
Sanitation should always be the first recourse against nematodes. Growers should select sites with no or low nematode populations, avoid the introduction of nematodes into the field, and scrupulously clean farm equipment used in different fields, both before and after using the equipment.
A common way that nematodes are introduced into fields is through infected plant material (transplants or tubers). It is generally difficult to recognize whether plant material is infected with nematodes. In the case of root-knot nematodes, the presence of galls or knots on roots of transplants or tubers is a telltale sign, but nematode galls can easily be overlooked when they are small or when roots are covered with soil. For most other nematodes, no real diagnostic root symptoms can be observed on planting material, and proper diagnosis will need to be done at a nematology lab.
Frequent applications of organic amendments (animal and green manures, compost, etc.) will increase soil organic matter and microbial activity while stimulating natural enemies that may reduce the damage caused by plant-parasitic nematodes.
Crop rotation with plants that are poor hosts for nematodes could be another option for Asian vegetable growers, but not enough is known now on the nematode host status of Asian vegetables to make good recommendations. Planting cover crops in between vegetable crops is a widely adopted nematode-management strategy. For managing root-knot nematodes, sunn hemp and sorghum-sudangrass are recommended as cover crops since they are known to be poor hosts (Bui and Desaeger 2021).
References
Abad, P., J. Gouzy, J. M. Aury, P. Castagnone-Sereno, E. G. Danchin, E. Deleury, L. Perfus-Barbeoch, V. Anthouard, F. Artiguenave, V. C. Blok, and M. C. Caillaud. 2008. “Genome Sequence of the Metazoan Plant-Parasitic Nematode Meloidogyne incognita.” Nature Biotechnology 26:909–915. https://doi.org/10.1038/nbt.1482
Baidoo, R., S. Joseph, T. M. Mengistu, J. A. Brito, R. McSorley, R. H. Stamps, and W. T. Crow. 2016. “Mitochondrial Haplotype-Based Identification of Root-Knot Nematodes (Meloidogyne spp.) on Cut Foliage Crops in Florida.” Journal of Nematology 48:193–202. https://doi.org/10.21307/jofnem-2017-027
Bernard, G. C., M. Egnin, and C. Bonsi. 2017. “The Impact of Plant-Parasiticnematodes on Agriculture and Methods of Control” in Nematology—Concepts, Diagnosis and Control, edited by M. M. Shah and M. Mahamood. IntechOpen Limited, London, United Kingdom. https://doi.org/10.5772/intechopen.68958
Brito, J. A., J. D. Stanley, M. L. Mendes, R. Cetintas, and D. W. Dickson. 2007. “Host Status of Selected Cultivated Plants to Meloidogyne mayaguensis in Florida.” Nematropica 37:65–72.
Brito, J. A., R. Kaur, R. Cetintas, J. D. Stanley, M. L. Mendes, E. J. McAvoy, T. O. Power, and D. W. Dickson. 2008. “Identification and Isozyme Characterisation of Meloidogyne spp. Infecting Horticultural and Agronomic Crops, and Weed Plants in Florida.” Nematology 10:757–766. https://doi.org/10.1163/156854108785787253
Brito, J. A., R. Kaur, R. Cetintas, J. D. Stanley, M. L. Mendes, T. O. Powers, and D. W. Dickson. 2010. “Meloidogyne spp. Infecting Ornamental Plants in Florida.” Nematropica 40:87–103.
Bui, H. X., and J. A. Desaeger. 2021. “Host Suitability of Summer Cover Crops to Meloidogyne arenaria, M. enterolobii, M. incognita and M. javanica. Nematology 1:1–9. https://doi.org/10.1163/15685411-bja10195
Bui, H. X, M. Gu, G. Riva, and J. A. Desaeger. 2022. “Meloidogyne spp. Infecting Asian Vegetables in Central Florida, USA.” Nematropica 52:56–63 .
Crow, W. T. 2020. “Nematode Management in the Vegetable Garden.” ENY-012. EDIS 2020. https://edis.ifas.ufl.edu/publication/NG005
Gu, M., H. X. Bui, J. A. Desaeger and W. Ye. 2021. “First Report of Meloidogyne enterolobii on Thai Basil in Florida, United States.” Plant Disease 105 (11): 3764. https://doi.org/10.1094/PDIS-02-21-0293-PDN
Joseph, S., T. Mekete, W. B. Danquah, and J. Noling. 2016. “First Report of Meloidogyne haplanaria Infecting Mi-Resistant Tomato Plants in Florida and Its Molecular Diagnosis Based on Mitochondrial Haplotype.” Plant Disease 100:1438–1445. https://doi.org/10.1094/PDIS-09-15-1113-RE
Kaiser, C. and M. Ernst. 2018. “Ethnic Vegetables: Asian.” CCD-CP-96. Lexington, KY: Center for Crop Diversification, University of Kentucky College of Agriculture, Food and Environment. Available: http://www.uky.edu/ccd/sites/www.uky.edu.ccd/files/asian.pdf
Liu, G. D. 2016. “Asian Vegetables Rapidly Emerging in Florida.” Vegetable and Specialty Crop News. https://vscnews.com/asian-vegetables-rapidly-emerging-florida/
Mathews, L. P., J. D. Peter, A. Shinsuke, and A. S. Hugh. 2022. Vegetable Production Handbook of Florida, 2022–2023 edition. CV100. EDIS 2022 (VPH). https://doi.org/10.32473/edis-cv292-2022
Noling, J. W. 1996. “Role of Soil Fumigants in Florida Agriculture.” in Fumigants: Environmental Fate, Exposure, and Analysis, edited by J. N. Seiber, J. E. Woodrow, M. V. Yates, J. A. Knuteson, N. L. Wolfe, and S. R. Yates. American Chemical Society. 12–24 https://doi.org/10.1021/bk-1997-0652.ch002
Table 1. Plant-parasitic nematodes associated with Asian vegetables grown in Central Florida according to our survey.