Introduction
Digestive health is an overlooked but vital component of the success of western honey bee (Apis mellifera) colonies. Viral, bacterial, fungal, and protozoal infections can harm bees’ digestive tracts and make it difficult for them to absorb nutrients and eliminate waste. Active management requires monitoring the health of individual honey bees as well as the health of the colony, providing adequate nutrition, and controlling for pests and diseases.
This publication supports beekeepers’ management by detailing how apiarists can detect and quantify infection by the organism Malpighamoeba mellificae, an amoeba that can cause disease in severely afflicted colonies.
This amoeba infects the Malpighian tubules, organs comparable to human kidneys. Malpighian tubules are responsible for removing waste from insects’ hemolymph (blood). The Malpighian tubules branch off the gut at the junction between the midgut and the hindgut.
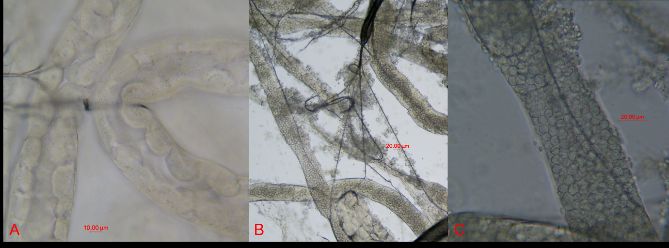
Credit: Etienne Tardif
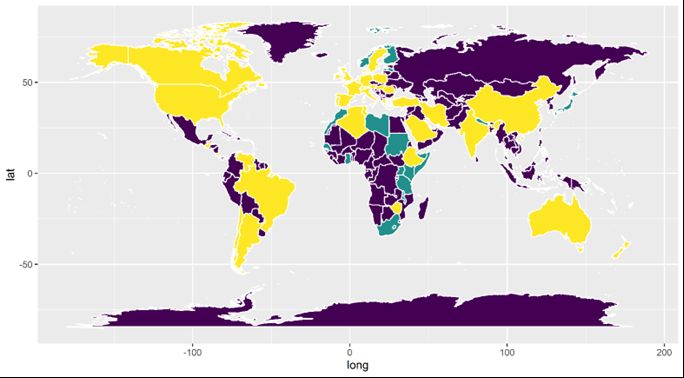
Due to the difficulty of detecting and diagnosing M. mellificae, its distribution and impact on honey bee health have been understudied. The current distribution of this organism is thought to be worldwide, occurring wherever its host, Apis mellifera, is present. Historically, the prevalence of M. mellificae is low in the regions where it has been found. However, new diagnostic techniques have emerged, among them nucleic acid-based molecular assays. These new techniques may be able to provide a more thorough evaluation of this parasite, allowing us to determine how common it is and giving us an idea of its significance for apiculture.
Credit: James Fulton, Florida Department of Agriculture and Consumer Services

Credit: Etienne Tardif
Worker honey bees are the most common caste to be infected with M. mellificae. This disease has not been found in honey bee drones and is seldom found in queens. Usually, queens become infected only when they are put in abnormal situations, such as caging. Transmission of the amoeba between bees likely occurs when bees are exposed to comb contaminated with feces from infected bees. This route of spread is called "fecal–oral.” Similarly, consumption of water contaminated with infected feces and trophallaxis (mouth-to-mouth food exchange) could be routes of exposure.
Based on beekeepers' observations, the prevalence of disease associated with M. mellificae (amoeba or amoebic disease) may be seasonal, with greater prevalence occurring during the winter or early spring. Although colony losses correlated to M. mellificae have been reported, it is common that cysts become undetectable in the previously infected colonies by late spring. Clinical signs that a honey bee colony may be infected with M. mellificae have been observed to resemble signs of a Nosema sp. infection. Nosema spp. are microsporidian parasites that, like M. mellificae, parasitize the honey bee gut. Clinical signs of both infections are similar: colony populations dwindle; bees experience dysentery (Figure 4); and bee lifespans are shortened. These similarities may come about because the two pathogens often occur together in a colony, and there may be additive or synergistic effects when both parasites are present.
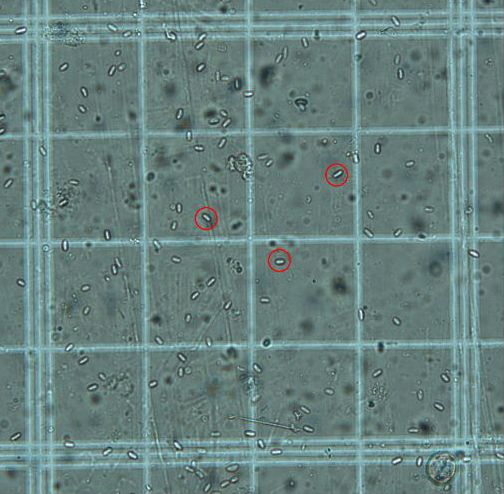
Credit: James Fulton, Florida Department of Agriculture and Consumer Services
Detection
If you observe an unusual increase in honey bee mortality and/or dysentery (excessive, unusually colored fecal material), you may then investigate for M. mellificae infection by using a compound light microscope to examine honey bee frass (feces).
To perform a visual inspection of bee feces to detect amoeba or Nosema spp., you will need the following:
- Compound light microscope with a 40x objective (400x magnification)
- Improved Neubauer hemocytometer with corresponding cover slip (optional) (Figure 5.)
- Glass coverslips
- Pipette (eye dropper)
- Forceps
- Water
- Gloves

Credit: James Fulton, Florida Department of Agriculture and Consumer Services
Then perform the following protocol:
- Collect ten live or dead bees from the front of each hive and place them into resealable bags labeled to identify the colony.
- Note: bees can be collected from anywhere in the hive, but selecting workers at the entrance may increase the likelihood of detecting the amoeba.
2. Add ten milliliters of distilled water to each bag, then crush to empty the bees' abdominal contents.
3. For simple detection (presence/absence), place a drop of liquid from the bag onto a microscope slide, apply a cover slip, and view with a light microscope. If you wish to quantify the contagion present to estimate the protozoal load, then you may use a hemocytometer to count M. mellificae spores in the same way you would use it to count Nosema spp. spores.
4. Spherical, colorless, translucent, 8–10 µm diameter cysts (Figure 6) will be visible with a light microscope at 400x magnification.
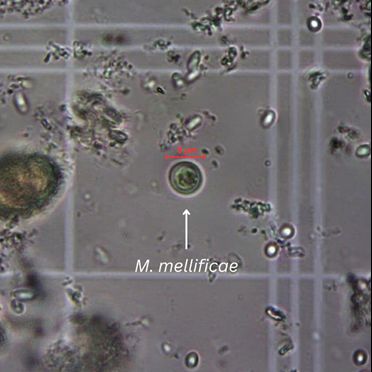
Credit: Marley Iredale, UF/IFAS Honey Bee Research and Extension Laboratory
Treatment
Currently, no standardized “economic threshold” is available for this amoeba. An economic threshold is the level of infection below which yields are unlikely to be affected and above which yield losses are likely. Additional research must be conducted to determine the number of amoebae necessary to cause disease in bees and what number indicates that management should be initiated. There are currently no commercially available treatments for amoebae. However, chemical treatments are continuously being developed, and other techniques for controlling amoebae are being tested. Management techniques that can be attempted if beekeepers suspect this infection in a colony include quarantining colonies and equipment, disinfecting equipment, and replacing contaminated comb. Common disinfection methods, such as dilute bleach, are likely effective in killing the amoeba in the environment; however, these methods have yet to be tested.
Final Remarks
Data useful for developing management solutions for M. mellificae are currently limited. Studies have shown how related amoeba species impair insects’ ability to excrete waste and toxins. However, more research is necessary to understand the effect M. mellificae may have on the honey bee. Until then, management of other hive pests and pathogens is recommended to limit the burden of amoebic infection on bees. Performing regular hive inspections, conducting alcohol mite washes, actively managing for V. destructor, and cleaning equipment between uses may help control the spread of or disease associated with this amoeba. The Ask IFAS publication How to Quantify Nosema Spores Infection Rate in a Honey Bee Colony (Mortenson et al. 2022) provides information about alcohol mite wash techniques. The method described above for detecting amoeba in honey bee samples is technically straightforward, requires limited equipment, and can be performed by anyone with access to the materials. Therefore, it provides a means by which beekeepers can start to detect and quantify this organism in their hives. In this way, we can expand our understanding of M. mellificae as a pathogen of honey bees.
References
Aydin, L., E. Gulegen, I. Cakmak, A. O. Girisgin, and H. Wells. 2006. “Relation Between Nosema and Chalkbrood Diseases, and its Implication for an Apiary Management Model.” Bulletin-Veterinary Institute in Pulawy. 50:471.
Bailey, L. 1968. “The Measurement and Interrelationships of Infections with Nosema apis and Malpighamoeba mellificae of Honey-Bee Populations.” Journal of Invertebrate Pathology 12 (2): 175–179. https://doi.org/10.1016/0022-2011(68)90174-2
Bailey, L., B. V. Ball, and J. N. Perry. 1983. “Association of Viruses with Two Protozoal Pathogens of the Honey Bee.” Annals of Applied Biology 103 (1): 13–20. https://doi.org/10.1111/j.1744-7348.1983.tb02735.x
Ben Abdelkader, F. 2020. “Situation of Beekeeping in North Africa.” Journal of Apitherapy and Nature 3 (1): 1–9. https://doi.org/10.35206/jan.719721
Berényi, O., T. Bakonyi, I. Derakhshifar, H. Köglberger, and N. Nowotny. 2006. “Occurrence of Six Honey Bee Viruses in Diseased Austrian Apiaries.” Applied and Environmental Microbiology 72:2414–2420. https://doi.org/10.1128/AEM.72.4.2414-2420.2006
Bessette, E., and B. Williams. 2022. “Protists in the Insect Rearing Industry: Benign Passengers or Potential Risk?” Insects 13 (5):482. https://doi.org/10.3390/insects13050482
Boncristiani, H., J. D. Ellis, T. Bustamante, J. Graham, C. Jack, C. B. Kimmel, A. Mortensen, and D. R. Schmehl. 2023. “World Honey Bee Health: The Global Distribution of Western Honey Bee (Apis mellifera L.) Pests and Pathogens.” Bee World 98 (1): 2–6, https://doi.org/10.1080/0005772X.2020.1800330
Costa, C. 2014. “Malattie da protozoi.” In Patologia e avversità dell’alveare, edited by Emanuele Carpana and Marco Lodesani. 205–210. Milan: Springer Milan. https://doi.org/10.1007/978-88-470-5650-3_7
Ellis, J. D., and P. A. Munn. 2005. “The Worldwide Health Status of Honey Bees.” Bee World 86 (4): 88–101, https://doi.org/10.1080/0005772X.2005.11417323
Evans, J. D., and R. S. Schwarz. 2011. “Bees Brought to Their Knees: Microbes Affecting Honey Bee Health.” Trends in Microbiology 19 (12): 614–620. https://doi.org/10.1016/j.tim.2011.09.003
Gutiérrez, B. D. V., and G. A. V. Bautista. 2016. “Diagnóstico de enfermedades parasitarias en abejas africanizadas Apis mellifera en el municipio de Marsella, Risaralda, Colombia.” Revista de Investigación Agraria y Ambiental 7(1). https://doi.org/10.22490/21456453.1618
Hussein, M. H. 2000. “Beekeeping in Africa: I. North, East, North-East and West African Countries.” Apiacta 1:32–48.
Kane, Terry Ryan, and Cynthia M. Faux. 2021. Honey Bee Medicine for the Veterinary Practitioner. John Wiley & Sons, Inc., NJ, U.S.A. https://doi.org/10.1002/9781119583417
Lannutii, L., F. N. Gonzales, M. J. Dus Santos, M. Florin-Christensen, and L. Schnittger. 2022. “Molecular Detection and Differentiation of Arthropod, Fungal, Protozoan, Bacterial and Viral Pathogens of Honeybees.” Veterinary Sciences 9 (5): 221. https://doi.org/10.3390/vetsci9050221
Liu, T. P. 1985. “Scanning Electron Microscope Observations on the Pathological Changes of Malpighian Tubules in the Worker Honeybee, Apis mellifera, Infected by Malpighamoeba mellificae.” Journal of Invertebrate Pathology 46 (2): 125–132. https://doi.org/10.1016/0022-2011(85)90140-5
Maaßen, A. 1916. “Über Bienenkrankheiten. Mitteil Kais Biol.” Reichsanstalt 16:51–58.
Malfroy, S. F., J. M. K. Roberts, S. Perrone, G. Maynard, and N. Chapman. 2016. “A Pest and Disease Survey of the Isolated Norfolk Island Honey Bee (Apis mellifera) Population.” Journal of Apicultural Research 55 (2): 202–211. https://doi.org/10.1080/00218839.2016.1189676
Matheson, A. 1993. “World Bee Health Report.” Bee World 74:176–212. https://doi.org/10.1080/0005772X.1993.11099183
Matheson, A. 1996. “World Bee Health Report.” Bee World (77) 1: 45–51, https://doi.org/10.1080/0005772X.1996.11099281
Mehlhorn, H. 2016. Encyclopedia of Parasitology 3rd edition: 400–402. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-662-43978-4_1839
Morse, R. A., and K. Flottum. 1997. Honey Bee Pests, Predators, and Diseases. A. I. Root Company.
Mortensen, Ashley N., Cameron J. Jack, Meghan McConnell, Liana Teigen, and James D. Ellis. 2022. How to Quantify Nosema Spores Infection Rate in a Honey Bee Colony. ENY-167. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/in1123
Noël, A., Y. L. Conte, and F. Mondet. 2020. “Varroa destructor: how does it harm Apis mellifera honey bees and what can be done about it?” Emerging Topics in Life Sciences 4 (1): 45–57. https://doi.org/10.1042/ETLS20190125
Rossi, M., S. R. Ott, and J. E. Niven. 2020. “Malpighamoeba infection Compromises Fluid Secretion and P-glycoprotein Detoxification in Malpighian Tubules.” Scientific Reports 10 15953 (2020). https://doi.org/10.1038/s41598-020-72598-z
Rossi, Marta. 2020. “Malpighian Tubule Function in Desert Locusts in Relation to Ecology, Disease and Plasticity.” University of Sussex. Thesis. https://hdl.handle.net/10779/uos.23477186.v1
Schäfer, M. O., J. Horenk, and C. Wylezich. 2022. “Molecular Detection of Malpighamoeba mellificae in Honey Bees.” Veterinary Sciences 9 (3): 148. https://doi.org/10.3390/vetsci9030148
Sinpoo, C., R. J. Paxton, T. Disayathanoowat, S. Krongdanga, and P. Chantawannakul. 2018. “Impact of Nosema ceranae and Nosema apis on Individual Worker Bees of the Two Host Species (Apis cerana and Apis mellifera) and Regulation of Host Immune Response.” Journal of Insect Physiology 105:1–8. https://doi.org/10.1016/j.jinsphys.2017.12.010
Waiselboim, A. J., and W. M. Farina. 2003. “Trophallaxis in Honeybees, Apis mellifera (L.), as Related to Their Past Experience at the Food Source.” Animal Behaviour 66 (4): 791–795. https://doi.org/10.1006/anbe.2003.2256
Yaeger, Robert G. 1996. “Protozoa: Structure, Classification, Growth, and Development.” Medical Microbiology. University of Texas Medical Branch at Galveston. ISBN 9780963117212.