Abstract
Vegetated coastal ecosystems, including mangroves, salt marshes, and seagrasses, store large amounts of carbon in plant biomass and underlying sediments, known as blue carbon. There is increasing interest among policymakers and natural resource managers in quantifying and monetizing the carbon stored in blue carbon ecosystems. However, carbon cycling in the marine and coastal environment is complex. This complexity and the logistical issues in accurately measuring blue carbon limit our ability to manage and restore blue carbon ecosystems as nature-based tools for climate change mitigation. This publication provides a general introduction to the processes that affect net carbon storage in blue carbon ecosystems and addresses implications for natural resource managers interested in quantifying net carbon storage.
This publication is a primer on blue carbon for coastal resource managers in Florida, including UF/IFAS Extension agents and Florida Sea Grant agents. We define blue carbon and describe ecological processes that affect carbon storage in Florida’s blue carbon ecosystems (mangroves, salt marshes, and seagrasses). We also identify challenges to accurately quantifying carbon storage.
Introduction to Carbon Storage in Natural Systems
Human-driven changes to the global carbon cycle have led to climate change, or long-term shifts in temperature and weather patterns. Combustion of fossil fuels and widespread land-use change have increased the amount of carbon dioxide (CO2) in the atmosphere. Elevated levels of CO2 in the atmosphere are the main driver of climate change and related consequences such as sea level rise and ocean acidification. Climate change is ongoing, and the Intergovernmental Panel on Climate Change (IPCC) 6th Assessment Report calls for immediate action to address CO2 emissions to prevent warming of 1.5°C above pre-industrial global average temperatures, beyond which dangerous impacts to Earth’s climate system are expected (Shukla et al. 2022). While technological and engineering advances in the industrial and energy sectors are necessary to solve this problem, there are also ways we can manage the environment to mitigate climate change as part of broader CO2 emissions reduction and removal strategies. Understanding how carbon flows through and is stored within the environment and in living things can help natural resource managers evaluate the climate outcomes of their management actions.
Carbon is essential to all living things. Organic carbon refers to carbon compounds produced by living things and incorporated within biomass. For example, plant photosynthesis converts CO2 into glucose (sugar), an organic carbon compound used for energy. Vegetation represents a large organic carbon sink, meaning it is a reservoir where more carbon is stored than is released. Inorganic carbon refers to carbon compounds found in nonliving things, such as gaseous CO2 in the atmosphere, mineral forms bound in rocks, and dissolved forms in water. The ocean is an example of a large inorganic carbon sink because it stores more CO2 than it emits. Human-driven transformations of organic carbon into inorganic carbon (i.e., CO2 gas) are the primary cause of rising CO2 levels in the atmosphere and, thus, climate change (Figure 1) (Wuebbles et al. 2017).
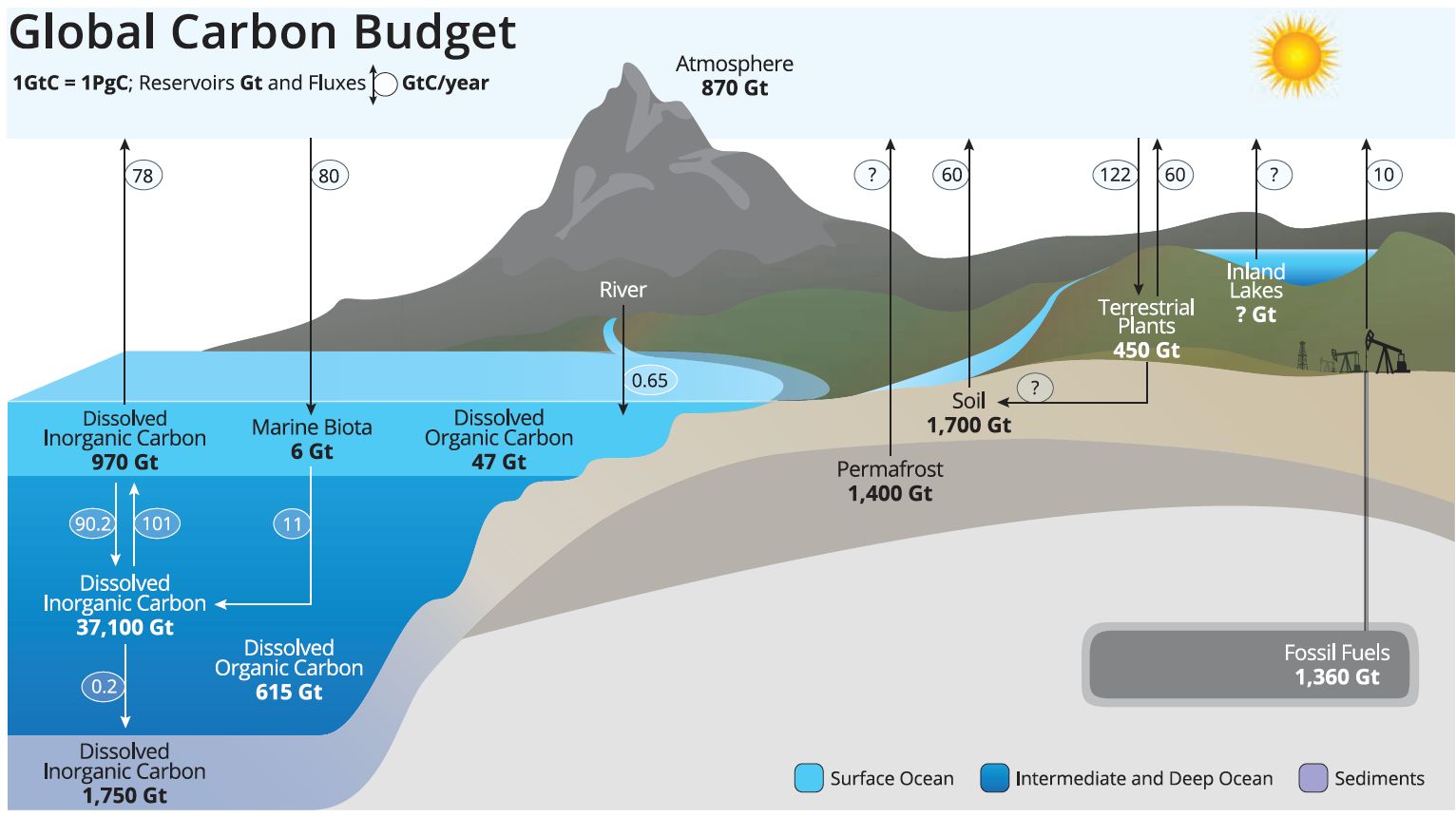
Credit: NOAA
Carbon stored in natural systems can be used to our advantage in dealing with climate change. We may want to preserve and promote natural processes that draw CO2 (inorganic carbon) out of the atmosphere and store it as organic carbon. For example, tree growth converts CO2 into plant biomass during reforestation. At the same time, we may want to discourage processes that remove organic carbon from storage and release it as CO2 into the atmosphere. For example, deforestation decreases the capacity of an area to store carbon, and subsequent burning or natural decay of the cleared vegetation releases CO2. For these reasons, highly productive ecosystems, like forests, play a major role in global carbon cycling. Therefore, we must thoughtfully manage forests and similar productive ecosystems to avoid unintentionally disrupting their carbon balance (Swingland et al. 2002; Canadell and Raupach 2008). Management actions that reduce or remove CO2 emissions, such as restoration, can compensate for emissions made elsewhere and are known as carbon offsets.
Our ability to use carbon stored in natural systems as a tool for climate change mitigation is limited because we do not fully understand the global carbon cycle or carbon dynamics within individual ecosystems, and these dynamics can change over time (see Caveats and Controversy). For example, climate change-driven fires and deforestation in the Amazon rainforest have changed some areas from carbon sinks to carbon sources (Gatti et al. 2021). Much like forests, coastal and marine vegetated ecosystems have become widely recognized as globally significant carbon stores; however, there are unique knowledge gaps preventing their use for climate change mitigation related to the complexity of carbon cycling in marine and coastal environments.
Blue Carbon in Mangroves, Salt Marshes, and Seagrass Meadows
We could gain minor climate benefits from optimizing the management of blue carbon (McLeod et al. 2011), which encompasses all the carbon stored in open and coastal oceans. There is strong evidence that coastal vegetated ecosystems (i.e., mangroves, salt marshes, and seagrasses) are particularly effective carbon sinks. Mangroves, salt marshes, and seagrasses store carbon for long periods of time and can be managed (i.e., protected or restored) to promote carbon storage (Duarte et al. 2013). A large fraction of the world’s blue carbon is stored in these vegetated coastal ecosystems, specifically in plant biomass, and buried in the underlying sediment (marine soil is typically referred to as sediment). Blue carbon in the sediment is estimated to accumulate at a rate of 131 Tg carbon (1 Tg = 1012 g) annually (Duarte et al. 2005)—approximately equal to the annual greenhouse gas emissions of Chile (European Commission 2023)—and is, therefore, a significant component of the global carbon budget. Importantly, sediment conditions in vegetated coastal ecosystems often prevent decomposition or decay of accumulated and buried carbon, which is why mangrove, salt marsh, and seagrass ecosystems can be such effective carbon sinks. Mangroves store an especially large amount of carbon, likely because mangrove trees are large, have a long life span, and contain decay-resistant woody tissue. Marshes and seagrasses contain considerably less carbon in plant biomass but still bury substantial quantities of carbon within the sediment. Compared to terrestrial forests, blue carbon ecosystems generally store less carbon in biomass but store disproportionately more carbon within sediments on a per-area basis (Figure 2). Carbon within sediments can be stored for long periods of time only if it is left undisturbed; if disturbed, it can rapidly re-enter the carbon cycle as CO2.
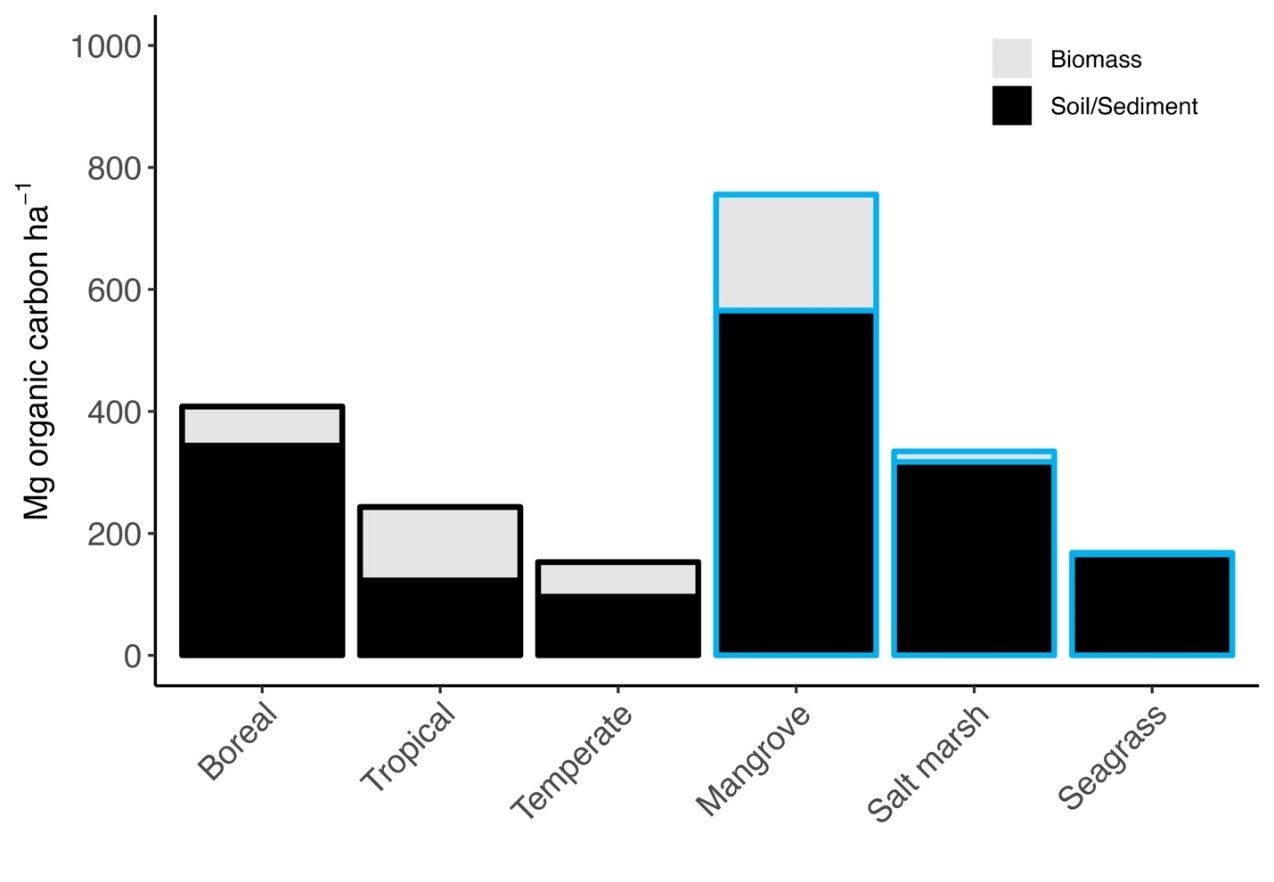
Credit: Data for terrestrial forests, mangroves and salt marshes, and seagrasses are from Watson et al. (2000), Alongi (2020), and Fourqurean et al. (2012), respectively.
There is growing policy interest in quantifying, protecting, and possibly monetizing blue carbon stores and utilizing blue carbon ecosystems as natural tools for climate change mitigation (Lovelock and Duarte 2019), as has been done for terrestrial systems, namely forests. However, more research is needed to understand the climate change mitigation potential of blue carbon. Global estimates of blue carbon accumulation rates and storage often come from site-specific measurements of plant and sediment carbon that are scaled by the area blue carbon ecosystems cover. Yet we do not know the global extent of some blue carbon ecosystems, especially for seagrasses, due to the difficulty of observing and mapping underwater vegetation. We first need to accurately measure blue carbon ecosystem distributions to understand their contributions to the global carbon cycle. Whether blue carbon ecosystems are truly carbon sinks is also uncertain because of the major assumption that carbon stored in coastal ecosystems is not outweighed by greenhouse gas emissions and other processes that release CO2 from these ecosystems (see Caveats and Controversy). More research is needed to understand how the management of blue carbon ecosystems fits within broader climate change mitigation strategies; however, research efforts within the last two decades have provided insights into the processes that lead to sediment organic carbon storage in marine and coastal environments.
The following sections will describe 1) how carbon accumulates and remains buried in coastal sediments, 2) environmental and biological factors that influence sediment carbon storage capacity, 3) causes and consequences of decreased storage capacity, 4) why blue carbon is under consideration as a climate mitigation tool, and 5) challenges to accurately quantifying blue carbon stores.
1. Carbon Accumulation, Burial, and Long-Term Storage
Organic carbon stored in sediments is the most stable form of blue carbon because it can remain in place for millennia. Mangroves, salt marshes, and seagrasses store such a large amount of carbon because they slow water movement and “trap” dead plant material and other forms of carbon-rich organic matter. The trapped carbon settles to the sediment surface where it accumulates over time and is eventually buried deeper and deeper within the sediment. The sediment-bound carbon in blue carbon ecosystems can be stored for millennia because marine sediment conditions discourage decomposition—a process that leads to the breakdown of organic matter into simpler organic and inorganic compounds, including CO2. Oxygen-poor sediments have slow rates of decomposition because microbes in low-oxygen conditions require much more energy to break down organic matter than microbes in high-oxygen conditions. Coastal plant root systems also play a major role in carbon storage by stabilizing sediments and allowing for accumulation (Chmura et al. 2003). Sediments with live roots are less likely to be resuspended by wave action or other physical disturbances. This publication focuses on carbon stored in sediments because plant biomass does not necessarily store carbon in as large quantities or for as long periods of time (except for mangrove trees): salt marsh and seagrass plants are relatively short-lived, and their tissue decomposes relatively quickly (Cebrian 1999).
Blue carbon storage is often quantified as stocks, or the mass of carbon per area. For sediments, carbon density within the top 1 m of sediments is typically measured, but in areas where sediments are shallower or difficult to sample, storage can be measured for shallower depths. For plant biomass, storage can be measured as the carbon content of aboveground and belowground biomass. Note that carbon storage, the amount of carbon contained within sediments or plant biomass, is different from carbon sequestration, the rate of sediment or plant uptake of CO2 from the atmosphere and incorporation into a more permanent reservoir (e.g., deeper, low-oxygen sediments or woody tissue) so it is not readily released. In general, carbon storage is easier to measure than carbon sequestration.
2. Drivers of Carbon Storage
Fundamentally, sediment carbon storage relies on 1) a carbon source and 2) environmental conditions that deliver carbon and allow sediment to accumulate. Carbon can be sourced from within a blue carbon ecosystem, such as decaying plant material, or from elsewhere, including organic matter carried by a river that flows to the coast. Hydrology (i.e., water movement) and bathymetry (i.e., water depth) are major factors that control sediment deposition, or the laying down of sediment carried by water. Sites that experience minimal wave energy and erosion are more likely to allow for sediment deposition and carbon accumulation. How much carbon a particular site accumulates depends on several factors related to carbon availability and sediment deposition rates (Figure 3).

Credit: Animal vector images are courtesy of Tracey Saxby, Integration and Application Network (ian.umces.edu/media-library).
Broad-scale site characteristics and processes impact carbon storage. Climate controls temperature and precipitation regimes across the world, influencing plant growth (Rosenzweig 1968) and soil decomposition rates (Davidson and Janssens 2006). Plant growth and soil decomposition constrain the amount of organic carbon produced and available for storage in both terrestrial and marine environments. Mangroves, salt marshes, and seagrasses are connected to nearby terrestrial ecosystems, so both oceanic processes and adjacent watershed characteristics influence the delivery of carbon and the likelihood of sediment deposition occurring within these ecosystems. Features of underwater terrain, such as the slope of a continental shelf, can influence wave energy, currents, tidal regime, and water depth—factors that control how well a site is sheltered from or exposed to water movement. Land-based runoff and river flow carry sediments and nutrients, which can directly and indirectly influence carbon storage. Delivery of fine-grained sediments to blue carbon ecosystems aids in the accumulation of organic carbon, but too much sedimentation can smother and kill seagrasses and potentially lead to the destabilization of underlying sediments and the resuspension of stored carbon. Nutrient enrichment can increase the productivity of blue carbon ecosystems and indirectly enhance carbon storage by increasing organic matter available for burial. At the same time, excessive nutrients can compromise the ability of salt marsh plants to stabilize sediments by redirecting growth into aboveground rather than belowground tissues (Deegan et al. 2012). The spatial arrangement of blue carbon ecosystems within a seascape also affects carbon storage: the level of connectedness among blue carbon ecosystems can influence storage because organic matter produced in one ecosystem may be exported to and stored within another (Krause et al. 2022).
At smaller spatial scales, local plant and animal populations can influence how effectively mangroves, salt marshes, and seagrasses trap and bury organic carbon. Plants can influence localized water movement, which in part determines whether a site is likely to experience sediment deposition or erosion. Mangroves, salt marshes, and seagrasses can all slow water movement and encourage deposition, though submerged vegetation (i.e., seagrasses) generally have smaller effects on water movement than emergent vegetation (i.e., marshes and mangroves). Grazing by larger herbivores and burrowing by infauna that disturb and oxygenate sediments can lead to carbon loss because high oxygen levels are more favorable to decomposition (Dahl et al. 2021). For example, crab burrowing can lead to soil carbon loss in mangroves (Pülmanns et al. 2014) and salt marshes (Martinetto et al. 2016). At even smaller spatial scales, sediment oxygen conditions and microbial activity control how much carbon that is deposited on the sediment surface will persist and be buried in the long term.
These aforementioned drivers ultimately determine the amount of carbon available for storage and whether site characteristics promote sediment deposition and carbon accumulation. This is not an exhaustive list of drivers, and drivers also interact, which is why carbon storage not only varies across ecosystems but also within ecosystems, sometimes at relatively small spatial scales. Several of these drivers are beyond the control of coastal managers, so environmental context is important to consider when managing an ecosystem for carbon storage.
3. Loss and Recovery of Carbon Stocks
Loss of a blue carbon ecosystem typically results in diminished sediment carbon storage because plants are no longer present to stabilize the sediments. Ecosystem removal can result from natural causes, such as seagrass die-off from disease or extreme temperature events like heat waves. Human activities, including the conversion of mangroves or marshes for development or aquaculture, can also remove or alter blue carbon ecosystems. Disturbances can diminish carbon storage even if vegetation persists; for example, storms and other weather events can cause scouring and erode carbon storage in surface sediments while leaving plants intact. It follows that less disturbed sites (or regions) that maintain consistent or stable levels of total vegetation cover over time tend to store more carbon. In Cedar Key, Florida, seagrass meadows stored the most carbon in surface sediments, where seagrass cover levels were consistent over the past 14 years (Bijak et al. 2023). If blue carbon ecosystems are lost, carbon stocks can be returned to pre-disturbance levels via natural recovery and restoration, though it takes years to decades. In Tampa Bay, Florida, the amount of carbon stored in created salt marshes did not approach storage levels in natural ecosystems until a decade or more post-restoration (Dontis et al. 2020).
In some cases, the loss of blue carbon ecosystems can be compensated by the natural expansion of another blue carbon ecosystem nearby. For example, losses and gains in ecosystem types, such as coastal forests, salt marshes, and seagrasses, offset one another to prevent major changes in regional carbon storage in a Virginia estuary (Smith et al. 2023). However, compensation by natural expansion is not likely or possible in all places, especially along developed coastlines where blue carbon ecosystems are threatened by sea level rise but cannot retreat landward.
4. Carbon Storage as Climate Change Mitigation
At the very least, protecting blue carbon ecosystems prevents an additional source of CO2 from entering the atmosphere because the removal of mangroves, salt marshes, and seagrasses can leave underlying sediment organic carbon more vulnerable to decomposition (Lovelock et al. 2017). In this way, blue carbon ecosystem protection can be considered a climate change mitigation strategy. Mitigation beyond simply preventing further unrealized emissions relies on the idea that restoring and creating new blue carbon ecosystems may help draw down CO2 from the atmosphere. However, it takes a long time (years to decades) to restore carbon stocks to pre-disturbance levels. Realistically, blue carbon ecosystem restoration or creation projects are unlikely to have more than a minor impact on climate change and will be most effective as a small part of broader strategies to reduce CO2 emissions and remove CO2 from the atmosphere. Even so, there are merits to considering carbon-accounting schemes as part of valuing ecosystems because protection and restoration of blue carbon ecosystems provide several other ecosystem services. It is also important to note that best practices for blue carbon ecosystem restoration are still in the research and development phase. Restoration success rates can vary considerably, and success rates are higher for salt marshes (64.8%) and mangroves (51.3%) than for seagrasses (38.0%) worldwide (Bayraktarov et al. 2016). Further, several components of the carbon cycle must be accounted for to demonstrate that blue carbon ecosystems are carbon sinks, requiring significant time and resources.
5. Caveats and Controversy
The balance of carbon stored in sediments and plant tissue versus emitted as CO2 in mangroves, salt marshes, and seagrasses is rarely quantified, so it is not clear whether these ecosystems are truly net carbon sinks. It is difficult to assess carbon sink capacity because carbon cycling is complex in blue carbon ecosystems. In addition to storing carbon, blue carbon ecosystems enhance organic carbon cycling, which results in the production of CO2 or other greenhouse gases that contribute to warming—including nitrous oxide (N2O) and methane (CH4)—through plant, animal, and microbial metabolism. Based on available estimates, mangroves, salt marshes, and seagrasses are greenhouse gas sinks globally: on average, they store more CO2 (or equivalent amounts of CH4 and N2O in terms of warming potential) than they emit (Rosentreter et al. 2023). However, greenhouse gas emissions differ within and across blue carbon ecosystems, and it is not yet clear which environmental and other factors drive this variability.
Additionally, inorganic carbon cycling can influence whether blue carbon ecosystems are net carbon sinks. Blue carbon ecosystems support a variety of organisms, often in higher abundances than in adjacent unvegetated ecosystems. Calcifying (e.g., shell-forming) organisms living in blue carbon ecosystems influence inorganic carbon cycling through calcification—a chemical reaction that extracts calcium and bicarbonate ions from seawater to form calcium carbonate and, in the process, releases CO2. In some cases, the amount of CO2 produced via calcification can exceed the amount of organic carbon buried in sediments and fixed in plant biomass. According to one study in Florida Bay, a seagrass meadow emitted more CO2 than it stored in sediments or plants, and about 95% of the emitted CO2 came from calcifying algae (Van Dam et al. 2021). In this case, the seagrass meadow was considered a carbon source rather than a carbon sink. CO2 emissions from calcification are not typically included in carbon storage assessments, so it is unclear how often calcification diminishes net carbon storage.
On the other hand, blue carbon ecosystems can also encourage calcified material in the sediments to dissolve, which has a net positive effect on carbon storage. Mangrove, salt marsh, and seagrass roots create acidic conditions in the sediment that can cause calcified material from either the underlying bedrock or shells to dissolve. Dissolution consumes CO2 and therefore increases ecosystem carbon storage. Blue carbon sediments are also often low in oxygen, where microbes must use anaerobic respiration to break down organic matter. Anaerobic respiration leads to the production of alkalinity, or bicarbonate and similar ions that ‘neutralize’ or consume CO2. Alkalinity production can therefore be an important process that potentially increases net carbon storage capacity but is rarely quantified in blue carbon ecosystems (Santos et al. 2021). In sum, net carbon storage assessments must consider how additional greenhouse gas emissions and inorganic carbon cycling (i.e., calcification, dissolution, and alkalinity production) may store or release carbon in addition to measuring the organic carbon stored in sediments and plants.
The complexity of carbon cycling within blue carbon ecosystems should make clear that assessing whether a specific location is a net sink or source of carbon at a specific point in time is challenging. Appropriate measurement scales for carbon cycling are difficult to identify because these processes occur at different time scales. For example, organic carbon stocks accumulate slowly as sediment accumulates over time (as slow as < 1 mm yr-1), while greenhouse gases are often measured by the order of hourly fluxes. Further, the boundaries of blue carbon ecosystems are fluid, enabling different forms of carbon to move easily between ecosystems, away from the ecosystem of origin. In some cases, blue carbon ecosystems promote storage beyond their footprint but do not actually store more carbon than adjacent bare areas. For example, dead seagrass material can be exported to and buried within marsh sediments (Ward et al. 2021; Krause et al. 2022), and dead mangrove material can be buried in sediments several kilometers offshore (Boto and Wellington 1988). Such contributions to carbon burial in other ecosystems are not often included in blue carbon estimates.
Do macroalgae beds and shellfish reefs store carbon?
Technically, macroalgae and shellfish reefs may be considered blue carbon ecosystems if they store carbon in biomass or underlying sediments, but they cannot be included in climate change mitigation strategies until we learn more about their ability to store carbon over the long term.
There is ongoing discussion as to whether macroalgae beds are long-term carbon sinks. The bulk of carbon stored in macroalgae beds is within algal biomass, not sediments, because macroalgae beds generally have lower sedimentation rates than traditional blue carbon ecosystems. At this point in time, high-biomass macroalgae ecosystems such as kelp forests are thought of as carbon sinks because they typically store more carbon in algal tissue than the kelp community, including microbes and fauna, emits as CO2 through respiration (Pessarrodona et al. 2023). Smaller and calcifying macroalgae that are common in Florida are less likely to be carbon sinks. Similar to plant biomass in other blue carbon ecosystems, there is a lot of uncertainty about the ultimate fate of algal biomass: it can be decomposed, consumed, or exported to the open ocean. Further, macroalgae beds can be very ephemeral, often appearing and disappearing over short time periods (e.g., annual cycles; Castorani et al. 2015), so assessing long-term carbon storage within these ecosystems is particularly challenging.
Shellfish reefs can accumulate organic carbon by filtering organic matter from the water column and excreting wastes known as biodeposits onto the sediment surface. If carbon-rich biodeposits are buried over time in low-oxygen sediments, long-term carbon storage can be achieved at levels similar to carbon storage found in mangroves, salt marshes, and seagrasses. However, carbon storage assessments must also consider that shellfish reefs release CO2 when the shellfish respire and form their shells (via calcification). Some argue the burial of shell material in reef sediments can be considered a long-term inorganic carbon sink. Shell material composed of inorganic carbon is unlikely to erode or dissolve when buried in deep, low-oxygen sediments and can therefore remain in place indefinitely. Yet CO2 immediately released into the water column during calcification when new shells are created may quickly diffuse into the atmosphere and, therefore, has stronger climate impacts in the near term. Shellfish reefs are estimated to be a small net carbon source globally, while the removal or excavation of shellfish reefs that exposes carbon-rich sediments to oxygen would likely release large amounts of carbon (Fodrie et al. 2017). As is the case for blue carbon ecosystems, shellfish reefs should be protected to prevent further emissions, but the climate impacts of reef restoration and aquaculture will be site-specific and costly to measure.
Conclusions
If we achieve a better understanding of blue carbon dynamics, we can prioritize ecosystems that warrant protection and identify best management practices to maintain their carbon stocks. It may be possible to enhance coastal carbon storage through the restoration of mangroves, salt marshes, and seagrasses, though at scale, the impact on climate change would be limited. More research is needed to understand the blue carbon potential of other ecosystems, like natural shellfish reefs and macroalgae beds, and for shellfish and algae aquaculture operations. Forging ahead, managers should be aware that carbon accounting is difficult and expensive, and though blue carbon offset standards have recently been published, blue carbon credit programs and markets are still in their infancy.
While the science of quantifying the net carbon balance of blue carbon ecosystems is not yet settled, managers can take steps to assess carbon storage or design projects with the goal of enhancing carbon storage in mangroves, salt marshes, and seagrasses. In any blue carbon assessment, it is important to account for carbon that may be gained or lost through processes that are difficult and costly to measure, including greenhouse gas emissions and inorganic carbon cycling (i.e., calcification, dissolution, and alkalinity production). When selecting project sites for restoration, managers should carefully consider how the environment, especially water movement, may affect sediment and carbon accumulation. There are methods to coarsely estimate sediment and plant carbon stocks that are inexpensive and do not require access to specialized laboratory equipment. For example, plant carbon stocks can be roughly estimated by multiplying biomass by values for carbon content reported from past studies. Yet more rigorous and standardized protocols must be followed to receive official recognition for blue carbon offsets from restoration or other projects. The following resources provide 1) detailed methods for measuring blue carbon stocks and CO2 emissions and 2) procedures to demonstrate the blue carbon offset potential of ecosystem restoration and related management actions:
- Howard, J., S. Hoyt, K. Isensee, E. Pidgeon, and M. Telszewski, eds. 2014. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows. Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature. https://www.thebluecarboninitiative.org/manual
- Emmer, I., B. Needelman, S. Emmett-Mattox, et al. 2023. Methodology for Tidal Wetland and Seagrass Restoration. Verified Carbon Standard, VM0033 v2.1. https://verra.org/methodologies/vm0033-methodology-for-tidal-wetland-and-seagrass-restoration-v2-1/
Glossary
alkalinity: Water’s ability to neutralize acid; alkalinity reacts with CO2 in seawater to form compounds that are not easily released into the atmosphere and, in this way, effectively consumes CO2
blue carbon: Carbon stored in marine and coastal environments, including in living biomass and organic matter buried within sediments, typically for the long term (i.e., decades to millennia)
calcification: The process by which marine organisms form calcium carbonate, for example, in mollusk shells or coral skeletons; during this process, CO2 is produced
carbon offsets: Actions taken to reduce CO2 emissions or increase carbon storage to compensate for CO2 emissions made elsewhere
carbon sequestration: Rate of CO2 uptake in plants (biologic) but also in geologic formations or through industrial processes
carbon sink: A reservoir that absorbs more carbon from the atmosphere than it releases
carbon storage: The total amount of carbon stored within the plants and soils of an ecosystem, such as a forest
decomposition: The breakdown of complex organic molecules into simpler organic and inorganic molecules
inorganic carbon: Carbon compounds that lack carbon-hydrogen bonds and are mostly found in nonliving parts of ecosystems (i.e., rocks, water, and sediments)
organic carbon: Carbon compounds primarily made of carbon and hydrogen and are mostly found in living parts of ecosystems (i.e., within organisms)
sediment deposition: The settling of sediment carried by water (or wind)
References
Alongi, D. M. 2020. “Carbon Balance in Salt Marsh and Mangrove Ecosystems: A Global Synthesis.” JMSE 8 (10): 767. https://doi.org/10.3390/jmse8100767
Bayraktarov, E., M. I. Saunders, S. Abdullah, et al. 2016. “The Cost and Feasibility of Marine Coastal Restoration.” Ecological Applications 26 (4): 1055–1074. https://doi.org/10.1890/15-1077
Bijak, A. L., L. K. Reynolds, and A. R. Smyth. 2023. “Seagrass meadow stability and composition influence carbon storage.” Landscape Ecology 38. https://doi.org/10.1007/s10980-023-01700-3
Boto, K. G., and J. T. Wellington. 1988. “Seasonal Variations in Concentrations and Fluxes of Dissolved Organic and Inorganic Materials in a Tropical, Tidally-Dominated, Mangrove Waterway.” Marine Ecology Progress Series 50: 151–160. https://doi.org/10.3354/meps050151
Canadell, J. G., and M. R. Raupach. 2008. “Managing Forests for Climate Change Mitigation.” Science 320 (5882): 1456–1457. https://doi.org/10.1126/science.1155458
Castorani, M. C. N., D. C. Reed, F. Alberto, et al. 2015. “Connectivity structures local population dynamics: A long-term empirical test in a large metapopulation system.” Ecology 96 (12): 3141–3152. https://doi.org/10.1890/15-0283.1
Cebrian, J. 1999. “Patterns in the Fate of Production in Plant Communities.” American Naturalist 154 (4): 449–468. https://doi.org/10.1086/303244
Chmura, G. L., S. C. Anisfeld, D. R. Cahoon, and J. C. Lynch. 2003. “Global Carbon Sequestration in Tidal, Saline Wetland Soils.” Global Biogeochemical Cycles 17 (4). https://doi.org/10.1029/2002GB001917
Dahl, M., M. Björk, and M. Gullström. 2021. “Effects of Seagrass Overgrazing on Sediment Erosion and Carbon Sink Capacity: Current Understanding and Future Priorities.” Limnology and Oceanography Letters 6 (6): 309–319. https://doi.org/10.1002/lol2.10211
Davidson, E. A., and I. A. Janssens. 2006. “Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change.” Nature 440: 165–173. https://doi.org/10.1038/nature04514
Deegan, L. A., D. S. Johnson, and R. S. Warren, et al. 2012. “Coastal Eutrophication as a Driver of Salt Marsh Loss.” Nature 490: 388–392. https://doi.org/10.1038/nature11533
Dontis, E. E., K. R. Radabaugh, A. R. Chappel, C. E. Russo, and R. P. Moyer. 2020. “Carbon storage increases with site age as created salt marshes transition to mangrove forests in Tampa Bay, Florida (USA).” Estuaries and Coasts 43: 1470–1488. https://doi.org/10.1007/s12237-020-00733-0
Duarte, C. M., I. J. Losada, I. E. Hendriks, I. Mazarrasa, and N. Marbà. 2013. “The Role of Coastal Plant Communities for Climate Change Mitigation and Adaptation.” Nature Climate Change 3: 961–968. https://doi.org/10.1038/nclimate1970
Duarte, C. M., J. J. Middelburg, and N. Caraco. 2005. “Major Role of Marine Vegetation on the Oceanic Carbon Cycle.” Biogeosciences 2 (1): 1–8. https://doi.org/10.5194/bg-2-1-2005
European Commission. 2023. Data from “GHG Emissions of All World Countries.” EDGAR (Emissions Database for Global Atmospheric Research). https://edgar.jrc.ec.europa.eu/report_2023
Fodrie, F. J., A. B. Rodriguez, R. K. Gittman, et al. 2017. “Oyster Reefs as Carbon Sources and Sinks.” Proceedings of the Royal Society B—Biological Sciences 284 (1859). https://doi.org/10.1098/rspb.2017.0891
Fourqurean, J. W., C. M. Duarte, H. Kennedy, et al. 2012. “Seagrass Ecosystems as a Globally Significant Carbon Stock.” Nature Geoscience 5: 505–509. https://doi.org/10.1038/ngeo1477
Gatti, L. V., L. S. Basso, J. B. Miller, et al. 2021. “Amazonia as a Carbon Source Linked to Deforestation and Climate Change.” Nature 595: 388–393. https://doi.org/10.1038/s41586-021-03629-6
Krause, J. R., A. Hinojosa-Corona, A. B. Gray, et al. 2022. “Beyond habitat boundaries: Organic matter cycling requires a system-wide approach for accurate blue carbon accounting.” Limnology and Oceanography 67 (S2): S6–S18. https://doi.org/10.1002/lno.12071
Lovelock, C. E., T. Atwood, J. Baldock, et al. 2017. “Assessing the Risk of Carbon Dioxide Emissions from Blue Carbon Ecosystems.” Frontiers in Ecology and the Environment 15 (5): 257–265. https://doi.org/10.1002/fee.1491
Lovelock, C. E., and C. M. Duarte. 2019. “Dimensions of Blue Carbon and Emerging Perspectives.” Biology Letters 15 (3). https://doi.org/10.1098/rsbl.2018.0781
Martinetto, P., D. I. Montemayor, J. Alberti, et al. 2016. “Crab Bioturbation and Herbivory May Account for Variability in Carbon Sequestration and Stocks in South West Atlantic Salt Marshes.” Frontiers in Marine Science 3. https://doi.org/10.3389/fmars.2016.00122
McLeod, E., G. L. Chmura, S. Bouillon, et al. 2011. “A Blueprint for Blue Carbon: Toward an Improved Understanding of the Role of Vegetated Coastal Habitats in Sequestering CO2.” Frontiers in Ecology and the Environment 9 (10): 552–560. https://doi.org/10.1890/110004
Pessarrodona, A., R. M. Franco-Santos, L. S. Wright, et al. 2023. “Carbon Sequestration and Climate Change Mitigation Using Macroalgae: A State of Knowledge Review.” Biological Reviews 98 (6): 1945–1971. https://doi.org/10.1111/brv.12990
Pülmanns, N., K. Diele, U. Mehlig, and I. Nordhaus. 2014. “Burrows of the semi-terrestrial crab Ucides cordatus enhance CO2 release in a North Brazilian mangrove forest.” PLoS ONE 9 (10): e109532. https://doi.org/10.1371/journal.pone.0109532
Rosentreter, J. A., G. G. Laruelle, H. W. Bange, et al. 2023. “Coastal vegetation and estuaries are collectively a greenhouse gas sink.” Nature Climate Change 13. https://doi.org/10.1038/s41558-023-01682-9
Rosenzweig, M. L. 1968. “Net Primary Productivity of Terrestrial Communities: Prediction from Climatological Data.” The American Naturalist 102 (923): 67–74. https://doi.org/10.1086/282523
Santos, I. R., D. J. Burdige, T. C. Jennerjahn, et al. 2021. "The Renaissance of Odum’s Outwelling Hypothesis in 'Blue Carbon' Science.” Estuarine, Coastal and Shelf Science 255: 107361. https://doi.org/10.1016/j.ecss.2021.107361
Shukla, P. R., J. Skea, R. Slade, et al., eds. 2022. Climate Change 2022: Mitigation of Climate Change. Working Group III Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. https://www.ipcc.ch/report/ar6/wg3/downloads/report/IPCC_AR6_WGIII_FullReport.pdf
Smith, A. J., K. McGlathery, Y. Chen, et al. 2023. “Compensatory mechanisms absorb regional carbon losses within a rapidly shifting coastal mosaic.” Ecosystems 27. https://doi.org/10.1007/s10021-023-00877-7
Swingland, I. R., E. C. Bettelheim, J. Grace, et al. 2002. “Forests, Carbon and Global Climate.” Philosophical Transactions of the Royal Society A—Mathematical, Physical and Engineering Sciences 360 (1797): 1567–1591. https://doi.org/10.1098/rsta.2002.1020
Wuebbles, D. J., D. W. Faheh, K. A. Hibbard, D. J. Dokken, B. C. Stewart, and T. K. Maycock, eds. 2017. Climate Science Special Report: Fourth National Climate Assessment, Vol. 1. U.S. Global Change Research Program. https://science2017.globalchange.gov/downloads/CSSR2017_FullReport.pdf
Van Dam, B. R., M. A. Zeller, C. Lopes, et al. 2021. “Calcification-driven CO2 emissions exceed 'Blue Carbon' sequestration in a carbonate seagrass meadow.” Science Advances 7 (51): eabj1372. https://doi.org/10.1126/sciadv.abj1372
Ward, M., T. Hill, C. Souza C, et al. 2021. “Blue Carbon Stocks and Exchanges Along the Pacific West Coast.” Biogeosciences Discussions 1–36. https://doi.org/10.5194/bg-2021-27
Watson, R., I. Noble, B. Bolin, et al., eds. 2000. Summary for Policymakers: Land Use, Land-Use Change, and Forestry, presented at Intergovernmental Panel on Climate Change, Montreal, Canada, May 1–8, 2000. https://www.ipcc.ch/site/assets/uploads/2018/03/srl-en-1.pdf