Introduction
Florida has the unfortunate distinction of being the global epicenter for nonnative reptiles, due to the intentional or unintentional actions of people. The state’s mild climate, abundant rainfall, expansive areas modified by humans, numerous international ports of entry (sea and air), as well as a thriving exotic pet trade all contribute to reptile invasions. Historically, reptiles invaded Florida unintentionally, often as stowaways in shipments of cargo. The first documented reptile introduction to Florida was that of the brown anole (Anolis sagrei) in the late 1800s (Garman 1887). This small lizard likely arrived accidentally in a shipment of cargo originating from Cuba. Since then, more than 150 additional nonnative reptile species have been documented in Florida, the vast majority of which were brought here through the pet reptile trade (Krysko et al. 2016).
Although most introductions do not result in the establishment of breeding populations of nonnative reptiles, many do. Of those species that become established, some thrive and expand, eventually becoming invasive. We define an invasive species of reptile as one that a) is not native to a specific geographic area (in this case the state of Florida), b) was introduced by the intentional or unintentional actions of humans, and c) does or can cause harm to the environment, economy, or human quality of life (Iannone et al. 2020). A well-known example of an invasive reptile in Florida is the Burmese python. This large species of snake was imported for the exotic pet trade, and by way of escapes and/or purposeful releases of pets, became established in the Everglades. Scientific studies have shown invasive pythons have caused severe declines of native mammals in the Everglades (Dorcas et al. 2012; McCleery et al. 2015) and have introduced parasites affecting Florida’s native snakes (Miller et al. 2017; Farrell et al. 2019). Additionally, state and federal agencies have spent millions of dollars to manage additional impacts of pythons and prevent them from expanding their range farther north in the peninsula and south into the Florida Keys.
Here we summarize general knowledge about established chameleons in Florida. Although eight species (FWC Nonnative Reptile List) have been documented in the state, only three species were established and breeding in Florida as of 2022: veiled chameleon (Chamaeleo calyptratus), Oustalet’s chameleon (Furcifer oustaleti), and panther chameleon (Furcifer pardalis). This publication is one in a series showcasing a suite of commonly seen or unique introduced reptiles that are established in the state. The biology and impacts of some of these species are well known, whereas others are poorly studied. This publication and others in the series were produced by undergraduate students in the course Invasion Ecology of Amphibians and Reptiles, which was taught in fall semester 2020 at the University of Florida. The target audience for all the publications in the series is homeowners and other residents and visitors who are curious about Florida’s diverse wildlife. Our goal is to increase knowledge and raise awareness about the many introduced and invasive reptiles in Florida, as well as to motivate people to take action in the fight to curtail spread of introduced reptiles in the Sunshine State.
Identifying Chameleons
Chameleons are distinctive lizards both in body form and coloration. Common characteristics shared among chameleons are their prehensile and often curled tails, independently moving eyes, and grasping, opposable toes well-suited for climbing (Figure 1). When attempting to identify a chameleon, their ability to change color and pattern based on mood, stress levels, and external factors should be considered. The veiled chameleon (Chamaeleo calyptratus) is distinguished from the other two species by the very high and triangular “casque” or helmet-like structure on its head, which is taller than those of the Oustalet’s and panther chameleons (Figure 2). Adult veiled chameleons range from 12 to 18 inches in total length (i.e., including the tail) and adult males are larger than females (Krysko, Enge, and Moler 2019). Both male and female veiled chameleons are boldly colored. Males are typically green with broad vertical bands of yellow, green, or orange along the sides of their body crossed with horizontal bands of black, brown, or yellow. Females are light green with tan, orange, white, or yellow markings (Figure 3) and those carrying eggs may be darker with turquoise and yellow speckling. Recently hatched young are a pastel green.

Credit: Steve A. Johnson and Natalie Claunch, UF/IFAS

Credit: (Left to right) Madison Harman, Steve A. Johnson, and Natalie Claunch, UF/IFAS

Credit: Natalie Claunch, UF/IFAS
Oustalet’s chameleon (Furcifer oustaleti) has a shorter and rounder casque than the veiled chameleon. They are one of the largest chameleon species in the world, with adults growing to approximately 12 to 24 inches in total length; males are larger than females (Krysko, Enge, and Moler 2019). Unlike the more boldly marked veiled chameleon and panther chameleon, adult male Oustalet’s chameleons are typically dull gray or brown. Patterning may include large, dark-edged circles or indistinct vertical bands on their sides. (Figure 4). Females have a coloration of varying greens, reds, and yellows, which may range from solid colors to more intricate patterning. Those carrying eggs may develop red lines along their head and body (Smith et al. 2016).

Credit: Steve A. Johnson, UF/IFAS
The panther chameleon (Furcifer pardalis) is distinguished from the veiled chameleon by its shorter casque and from Oustalet’s chameleon by ridges extending over the eyes and snout, forming a shovel-like projection above its nose. Adults range from 12 to 22 inches, with males growing considerably larger than females (McGeogh 2016). The male panther chameleon displays a range of color far greater than the other two introduced chameleons. The color patterns of male panther chameleons differ based on geography in their native range of Madagascar, and several “locales” or color lineages have been introduced to Florida (Fieldsend et al. 2021). Bright blues, yellows, oranges, greens, pinks, and reds are common. They often have a white stripe along the side of their body, which may be solid or interrupted by vertical bars of color. Females are generally less striking in coloration with more muted shades of tan, pink, orange, or brown (Figure 5).

Credit: Natalie Claunch, UF/IFAS
Similar-looking Species
The three chameleon species may be confused with the brown basilisk (Basiliscus vittatus) due to the similarity of the chameleon’s head casques and the male brown basilisk’s crest. Brown basilisks are also not native to Florida but have been introduced to the state from their native range in Mexico, Central America, and South America (Krysko et al. 2006). Male brown basilisks have a large crest on the back of their heads (Figure 6), which inspired their name, meaning “little king” in Greek. They may have an additional crest that runs down their backs from the neck to the hind legs, which can resemble the spine-like scales along the backs of chameleons. The shape of the tail is a good feature to easily differentiate the chameleons from brown basilisks. The brown basilisk’s tail is long and thin, as compared to the chameleon’s tail, which is broader and often coiled. Brown basilisks have long, slender toes that are not opposable. Also, chameleons are cautious and slow-moving, even during an escape. Brown basilisks, like most other Florida lizards, are quick to flee if approached. If the lizard in question is fast, it is not a chameleon. For more information on brown basilisks in Florida, see the publication at this link (https://edis.ifas.ufl.edu/publication/UW497).

Credit: Steve A. Johnson, UF/IFAS
One of Florida’s native and widespread lizards, the green anole (Anolis carolinensis), is sometimes referred to as the “American chameleon” due to its ability to quickly change color from shades of brown to bright green while attempting to blend into its environment. Green anoles are not closely related to chameleons. Like true chameleons, however, they are usually found perched in vegetation. The green anole can be distinguished from a true chameleon by its slender head, long uncurled tail, and separated toes. All three of these body features are noticeably longer and more slender than those of a chameleon (Figure 7). Male green anoles also have a bright pink dewlap, or throat fan, that they extend as a territorial gesture or an attempt at attracting a female.

Credit: Steve A. Johnson, UF/IFAS
Native Range
Chameleons are native to Africa, Madagascar, southern Europe, and Asia. Each species has their own specific habitat requirements, but typically chameleons thrive in warm climates in habitats ranging from rainforest to desert. They are most common in woody areas with abundant trees and bushes. The veiled chameleon can be found in southwestern Saudi Arabia and the Republic of Yemen, occupying a variety of habitats including high and dry plateaus, forests, and river valleys. The native range of the Oustalet’s chameleon lies in Madagascar where it occupies both dry and humid habitats at low to high elevations. Panther chameleons are also from Madagascar and occur in the northern and eastern parts of the country where they are found frequently in forests along rivers and roads (Andreone, Guarino, and Randrianirina 2015). This species has accounted for almost 50% of all chameleons exported from Madagascar since the mid-1990s (Krysko, Enge, and Moler 2019).
Mode of Introduction to Florida
Chameleons have been exported from various countries through the pet trade since the 1800s but beginning in the 1970s the demand for chameleons increased significantly. From 1977 to 2001, the majority (~95%) of documented chameleon exports were from Madagascar, Tanzania, and Togo, while the United States dominated the import market (Carpenter, Rowcliffe, and Watkinson 2004). Chameleons have been introduced to Florida as a result of this global trade—the three species currently established in Florida were imported by the exotic pet industry. Some of these introductions are the result of accidental releases, and some individuals were released intentionally with the goal of establishing localized populations (Gillette et al. 2010). Florida’s warm and humid climate, especially in the southern half of the state, creates ideal conditions for introduced reptiles to survive and reproduce (Krysko et al. 2016).
Florida Range and Habitats
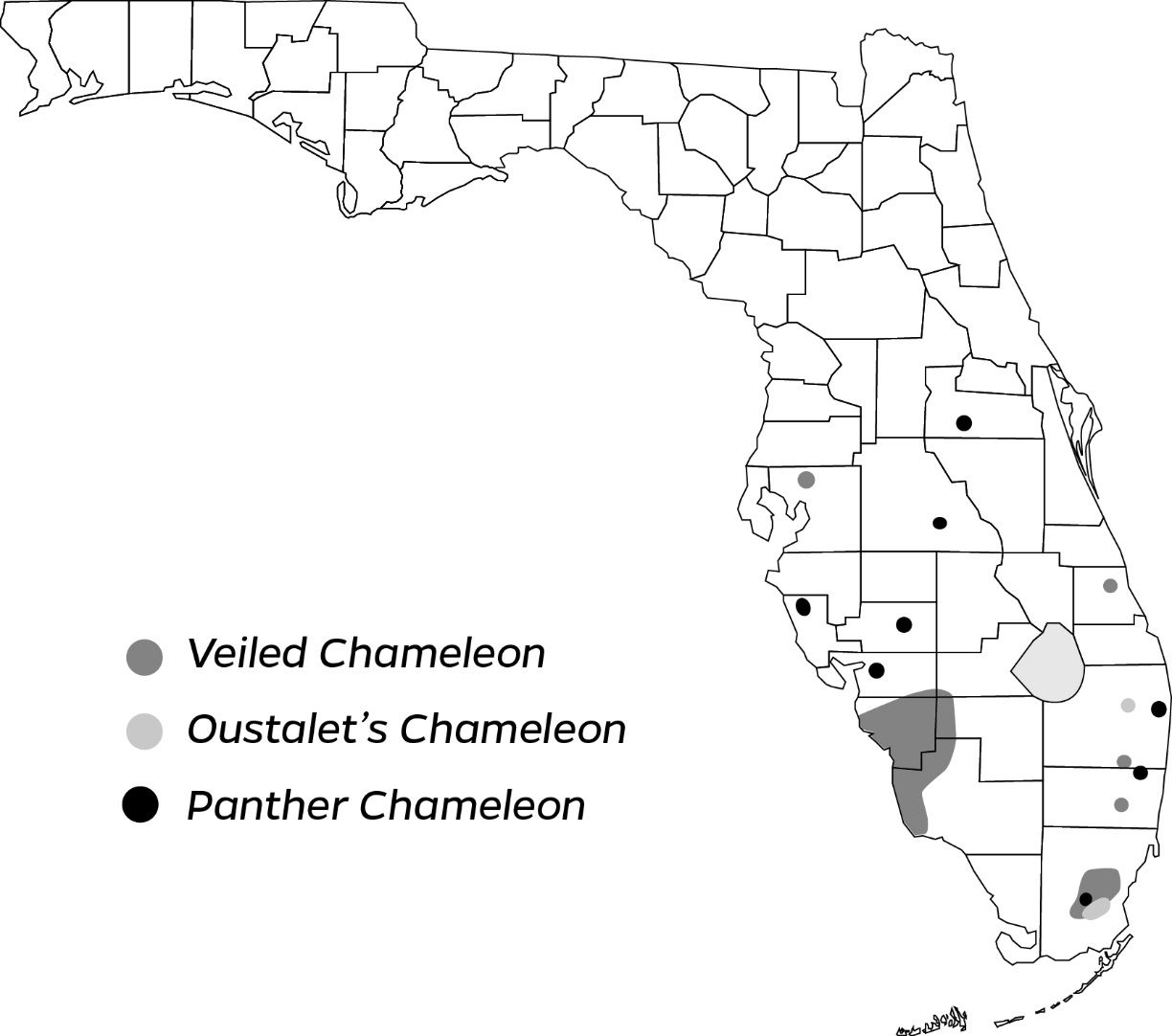
All three chameleon species are known to occur in isolated populations within the southern half of the Florida peninsula (Figure 8). They mainly occur in suburban settings and other human modified environments.
Credit: Tracy Bryant, UF/IFAS Communications
Oustalet’s chameleon was introduced to Florida before 2000 to avocado groves behind the facility of a former animal importer in Miami-Dade County (Gillette et al. 2010). Records from EDDMaps, a web-based mapping system used to document invasive species, indicate this population has remained active at least through 2017, when state surveys ceased (Rodgers et al. 2020). Several individuals have also been found in Palm Beach County. In Florida, Oustalet’s chameleons have been found in vegetation including avocado, gumbo-limbo, oak, lancewood, and white leadtree.
The panther chameleon was first documented in Florida in 2008 and appears to have established by 2012 (Krysko et al. 2016). Although little has been published about their use of habitats in the state, like other chameleons, panther chameleons are arboreal and likely use a variety of vegetation in which to rest and forage—they have been observed sleeping in gumbo-limbo trees (Rochford et al. 2013). They have been documented in numerous, broadly distributed locations throughout the peninsula. Although some are individual occurrences of single escaped pets, multiple individuals have been reported from a small area in Broward County that likely has a reproducing population (Rochford et al. 2013). Additionally, there appear to be other small but established populations in several other counties in the central peninsula (Fieldsend et al. 2021).
Of the three chameleon species, the veiled chameleon appears to be the most widespread in the state. This species was first documented in Florida in 2002 from Fort Myers (Lee County) in a vacant lot (Krysko, Enge, and King 2004) after introduction through the pet trade from 2000–2001. Additional introductions have been recorded in Hendry County (Enge 2008), Miami-Dade County (Gillette and Krysko 2012), and Broward County (Edwards et al. 2014). Based on EDDMaps and museum records, populations of this species are likely established in other counties. Veiled chameleons in Florida have been observed in vegetation including avocado, Brazilian pepper, castor bean, and carrotwood (Krysko, Enge, and Moler 2019; Metzger and Ginoza 2021).
Chameleons in Florida are not known to disperse far from their site of introduction, so most populations occur in or adjacent to urban areas, and there is little record of them invading natural areas. Populations are often self-sustaining and are notoriously complicated to track down. Existing populations that become known among reptile enthusiasts may decline or disappear through collection as pets. Meanwhile, new populations are illegally seeded in different areas for sustained collection. Due to their popularity in the pet trade, populations are often kept private by hobbyists for this purpose and detailed information on their distribution is not publicly available. The distribution map created for this publication (Figure 8) was compiled from public records and likely does not reflect the full extent of breeding occurrences for the three chameleon species in the state.
Chameleon Ecology
All three chameleon species are diurnal, occupying lower vegetation when active during the day and sleeping higher in vegetation at night. Chameleons are generally solitary, and males will maintain and defend territories from other males. They communicate through movement (e.g., head jerking) and visual signals by changing color. The veiled chameleon has also been documented using vibrations, which travel through vegetation, as a possible method of communication (Barnett, Cocroft, and Fleishman 1999). In their native range, veiled chameleons may regulate their body temperature by retreating to warmer refugia (e.g., crevices in rocks and holes in the ground) or by panting to relieve heat. They are likely to follow similar behavior patterns in Florida.
Chameleons primarily eat insects but also consume small vertebrates, including other lizards, small mammals, and birds. They are sit-and-wait predators who use their independently mobile eyes to spot prey and then rapidly project their sticky tongues to distances up to twice their body length to capture it. In Florida, Oustalet’s chameleons feed largely on moth larvae (Krysko et al. 2012) but will also eat small fruits (Takahashi 2008). Veiled chameleons eat flowers and plants as well.
The veiled, Oustalet’s, and panther chameleons all lay their eggs in holes in the ground, often at the base of trees. In captivity, the veiled chameleon lays 3–4 clutches (i.e., groups of eggs) per year with about 30–40 eggs per clutch. The eggs take 4–6 months to hatch, and hatchlings reach sexual maturity in as early as four months. The Oustalet’s chameleon lays an average of 42.3 eggs per clutch in Florida (Smith et al. 2016). These eggs hatch after roughly 10–12 months, and hatchlings become sexually mature in a year. The panther chameleon lays multiple clutches annually of 12–46 eggs that hatch within 7–8 months (Krysko, Enge, and Moler 2019).
Ecological Impacts
The current ecological impacts of Florida’s three introduced chameleons are poorly understood, though their greatest potential for ecological harm likely lies with their diet. A preliminary diet study of an Oustalet’s chameleon population in Florida revealed that moth larvae are a commonly consumed food item, but also recorded a brown anole (Anolis sagrei), another of Florida’s introduced reptiles (see the publication “Florida's Introduced Reptiles: Brown Anole (Anolis sagrei)“), in the stomach of one individual (Krysko et al. 2012). In its native range of Madagascar, an adult male Oustalet’s chameleon was observed with a dead bird in its mouth, which it proceeded to swallow (Garcia and Vences 2002). Populations of introduced chameleons elsewhere may have impacts on imperiled species. In Hawaii for example, the invasive Jackson’s chameleon (Trioceros jacksonii xantholophus) was documented consuming one of several species of endangered tree snail (Chiaverano and Holland 2014).
Impacts to People and Pets
Chameleons may be considered a nuisance species by those who do not want them in their yards, as they may eat decorative garden plants. They may also hiss or deliver a strong bite when roughly handled. However, except for larger individuals, bites are unlikely to break skin, and chameleon bites are not toxic to humans or pets. The presence of chameleons in an area will often draw collectors, and there have been occurrences of collectors trespassing on private property at night when chameleons are most easily spotted and captured (Edwards et al. 2014).
Economic Impacts
Currently, there are no documented or suspected negative economic impacts of introduced chameleons in Florida beyond the routine costs of limited survey and control efforts.
How you can help!
Chameleon populations are notoriously difficult to document in the wild in Florida. If you observe a chameleon, especially outside of the shaded areas shown on the map in Figure 8, take a digital image of it and report your observation at IveGot1.org or through the free IveGot1 Smartphone app. You can also report photographed sightings to other species-reporting platforms such as iNaturalist, or to the Florida Museum of Natural History.
Captive chameleons are challenging to keep as pets. If you have a pet chameleon, or another exotic pet that you are no longer able to care for or do not want to keep, you can surrender it for adoption through the FWC’s Exotic Pet Amnesty Program: https://myfwc.com/wildlifehabitats/nonnatives/amnesty-program/. This program allows owners of exotic, nonnative pets (i.e., not livestock or domestic pets such as cats and dogs) to relinquish their pet at no cost or penalty. Surrendered pets are rehomed with experienced, pre-approved adopters. Please, DO NOT release unwanted exotic pet reptiles such as chameleons. This is unethical (the lizard may starve to death, be killed by a predator, or harm native species) and is also against the law in Florida.
Because they are a nonnative species, chameleons are not protected in Florida except by anti-cruelty laws. They can be humanely removed or killed on private property year-round with landowner permission. Captured chameleons cannot legally be released at other locations in Florida. Chameleons cannot be collected for sale without a permit. If you are not capable of safely removing chameleons from your property, please seek assistance from a professional nuisance wildlife trapper.
Additional Sources of Information
EDDMaps: https://www.eddmaps.org/
FWC Exotic Pet Amnesty Program: https://myfwc.com/wildlifehabitats/nonnatives/amnesty-program/
Chameleons—Everglades CISMA: https://www.evergladescisma.org/the-dirty-dozen/chameleons/
Literature Cited
Andreone, F., F. M. Guarino, and J. E. Randrianirina. 2005. “Life History Traits, Age Profile, and Conservation of the Panther Chameleon, Furcifer pardalis (Cuvier 1829), at Nosy Be, NW Madagascar.” Tropical Zoology 18:209–225. https://doi.org/10.1080/03946975.2005.10531221
Barnett, K. E., R. B. Cocroft, and L. J. Fleishman. 1999. “Possible Communication by Substrate Vibration in a Chameleon.” Copeia 1999:225–228. https://doi.org/10.2307/1447408
Carpenter, A. I., J. M. Rowcliffe, and A. R. Watkinson. 2004. “The Dynamics of the Global Trade in Chameleons.” Biological Conservation 120:291–301. https://doi.org/10.1016/j.biocon.2004.03.002
Chiaverano, L. M., and B. S. Holland. 2014. “Impact of an Invasive Predatory Lizard on the Endangered Hawaiian Tree Snail Achatinella mustelina: A Threat Assessment.” Endangered Species Research 24:115–123. https://doi.org/10.3354/esr00589
Dorcas, M. E., J. D. Willson, R. N. Reed, R. W. Snow, M. R. Rochford, M. A. Miller, W. E. Meshaka, Jr., et al. 2012. “Severe Mammal Declines Coincide with Proliferation of Invasive Burmese Pythons in Everglades National Park.” Proceedings of the National Academy of Sciences 197:2418–2422. https://doi.org/10.1073/pnas.1115226109
Edwards J. R., M. R. Rochford, F. J. Mazzotti, and K. L. Krysko. 2014. “New County Record for the Veiled Chameleon (Chamaeleo calyptratus Duméril and Bibron 1851), in Broward County, Florida, with Notes on Intentional Introductions of Chameleons in Southern Florida.” Reptiles & Amphibians 21:83–85. https://doi.org/10.17161/randa.v21i2.13997
Enge, K. M. 2008. “Geographic Distribution: Chamaeleo calyptratus (Veiled Chameleon).” Herpetological Review 39:367.
Farrell T. M., J. Agugliaro, H. D. S. Walden, J. F. X. Wellehan, A. L. Childress and C. M. Lind. 2019. “Spillover of Pentastome Parasites from Invasive Burmese Pythons (Python bivittatus) to Pygmy Rattlesnakes (Sistrurus miliarius), Extending Parasite Range in Florida, USA.” Herpetological Review 50:73–76.
Fieldsend, T. W., N. M. Claunch, B. T. Fridie, C. M. Goodman, M. E. A. Harman, K. L. Krysko, C. J. Raxworthy, C. M. Romagosa, and T. M. Collins. 2021. “Extreme Male Color Polymorphism Supports the Introduction of Multiple Native-Range Panther Chameleon (Furcifer pardalis) Lineages to Florida, USA.” Reptiles and Amphibians 28:257–261. https://doi.org/10.17161/randa.v28i2.15599
Garcia, G., and M. Vences. 2002. “Furcifer oustaleti (Oustalet’s Chameleon): Diet.” Herpetological Review 33:134–135.
Garman, S. 1887. “On West Indian Reptiles: Iguanidae.” Bulletin of the Essex Institute 19:25–50.
Gillette, C. R., and K. L. Krysko. 2012. “New County Record for the Veiled Chameleon, Chamaeleo calyptratus Duméril and Bibron 1851 (Sauria: Chamaeleonidae), in Florida.” Reptiles & Amphibians 19:130–131. https://doi.org/10.17161/randa.v19i2.13893
Gillette, C. R., K. L. Krysko, J. A. Wasilewski, G. N. Kieckhefer III, E. F. Metzger III, M. R. Rochford, D. Cueva, and C. Smith. 2010. “Oustalet’s Chameleon, Furcifer oustaleti (Mocquard 1894) (Chamaeleonidae), a Non-Indigenous Species Newly Established in Florida.” Reptiles & Amphibians 17:248–249.
Iannone, B. V, III, S. Carnevale, M. B. Main, J. E. Hill, J. B. McConnell, S. A. Johnson, S. F. Enloe, et al. 2020. “Invasive Species Terminology: Standardizing for Stakeholder Education.” Journal of Extension 58: Published digitally (https://archives.joe.org/joe/2020june/a3.php). Accessed January 2, 2022.
Krysko, K. L., K. M. Enge, and F. W. King. 2004. “The Veiled Chameleon, Chamaeleo calyptratus: A New Exotic Lizard Species in Florida.” Florida Scientist 67:249–253. https://www.jstor.org/stable/24321170?seq=1
Krysko, K. L., K. M. Enge, and P. E. Moler. 2019. Amphibians and Reptiles of Florida. University of Florida Press, Gainesville. 706 pp.
Krysko, K. L., C. R. Gillette, R. M. Reichart, L. P. Nuñez, N. T. Coutu, J. A. Wasilewski, K. M. Enge, and A. P. Borgia. 2012. “Preliminary Diet Analysis for the Non-Indigenous Oustalet’s Chameleon, Furcifer oustaleti (Mocquard 1894) (Squamata: Chamaeleonidae), in Southern Florida.” Reptiles & Amphibians 19:280–287. https://doi.org/10.17161/randa.v19i4.13926
Krysko, K. L., J. C. Seitzl, J. H. Townsend, and K. M. Enge. 2006. “The Introduced Brown Basilisk (Basiliscus vittatus) in Florida.” Iguana 13:25–30.
Krysko, K. L., L. A. Somma, D. C. Smith, C. R. Gillette, D. Cueva, J. A. Wasilewski, K. M. Enge, et al. 2016. New Verified Nonindigenous Amphibians and Reptiles in Florida through 2015, with Summary of over 152 Years of Introductions. Reptiles & Amphibians 23:110–143. https://doi.org/10.17161/randa.v23i2.14119
McCleery, R. A., A. Sovie, R. N. Reed, M. W. Cunningham, M. E. Hunter, and K. M. Hart. 2015. “Marsh Rabbit Mortalities Tie Pythons to the Precipitous Decline of Mammals in the Everglades.” Proceedings of the Royal Society B 282:1805 https://doi.org/10.1098/rspb.2015.0120
McGeogh, R. 2016. “Furcifer pardalis (Panther Chameleon) – A Brief Description and Details on Captive Husbandry. BEMS Reports 2:27–38. https://doi.org/10.5530/BEMS.2016.2.6
Metzger, E. F., and M. E. C. Ginoza. 2021. “New Distributional Records of Veiled Chameleon (Chamaeleo calyptratus Dumeril and Bibron 1951) in Palm Beach County, Florida.” Reptiles and Amphibians 28:558–559. https://doi.org/10.17161/randa.v28i3.15883
Miller, M. A., J. M. Kinsella, R. W. Snow, M. M. Hayes, B .G. Falk, R. N. Reed, F. J. Mazzotti, C. Guyer, and C. M. Romagosa. 2017. “Parasite Spillover: Indirect Effects of Invasive Burmese Pythons.” Ecology and Evolution 8:830–840. https://doi.org/10.1002/ece3.3557
Rochford, M. R., J. R. Edwards, P. L. Howell, J. K. Eckles, L. A. Barraco, L. L. Connor, M. J. Curtis, K. L. Krysko, and F. J. Mazzotti. 2013. “The Panther Chameleon, Furcifer pardalis (Cuvier 1929) (Chamaeleonidae), Another Introduced Chameleon Species in Florida.” Reptiles & Amphibians 20:205–207. https://doi.org/10.17161/randa.v20i4.13974
Rodgers, L., C. Mason, R. Brown, M. Kirkland, D. Bagiotti, P. Tipping, J. Nestler, et al. 2020. “Chapter 7: Status of Nonindigenous Species.” South Florida Environmental Report. South Florida Water Management District 1:1–72. https://apps.sfwmd.gov/sfwmd/SFER/2020_sfer_final/v1/chapters/v1_ch7.pdf
Smith, D., J. Vinci, C. V. Anderson, J. K. Eckles, F. Ridgley, and F. J. Mazzotti. 2016. “Observations on Nesting and Clutch Size in Furcifer oustaleti (Oustalet’s chameleon) in South Florida.” Southeastern Naturalist 15:75–88. https://doi.org/10.1656/058.015.sp808
Takahashi, H. 2008. “Fruit Feeding Behavior of a Chameleon Furcifer oustaleti: Comparison with Insect Foraging Tactics.” Journal of Herpetology 42:760–763. https://doi.org/10.1670/07-102R2.1