Artificial Reefs in Florida 101—why this fact sheet series and this specific fact sheet?
Increasingly, coastal managers and practitioners are placing artificial reefs in marine waters. Artificial reefs are expensive to deploy, but they are much more expensive to remove. This makes them relatively permanent habitat alterations. These long-lasting habitat alterations have measurable effects on fish, fishers, divers, fisheries, and the overall marine ecosystem and connected human socioeconomic system. It is critical to understand how artificial reefs “work” to make good decisions about future artificial reefs. There is scientific research on many aspects of artificial reefs, but this information is not always summarized and explained. In response to this need, we designed a 4-part series called Artificial Reefs 101:
- Part 1 describes why artificial reefs are built in the first place.
- Part 2 describes how artificial reefs affect fish ecology.
- Part 3 describes how artificial reefs affect users, mostly focusing on fishers.
- Part 4 describes how the overall fishery system (both fish and fishers) is affected by artificial reefs.
The Artificial Reefs 101 series provides a general overview for people seeking to better understand artificial reefs in Florida. It will also give additional background material for those wishing to dive deeper into the science behind artificial reefs. The Artificial Reefs 101 series complements existing publications about specific aspects of artificial reefs, such as how they are implemented (FA231), the ecological effects of artificial reefs on fish (SG100), and the economic benefits of artificial reefs (FE649). There is additional information available through the Florida Master Naturalist Program in the Marine Habitat Restoration Course.
This specific fact sheet describes the most common and well-studied ways artificial reefs affect fish populations. While artificial reefs may affect a large number of fish and other marine life, this series and fact sheet primarily focus on fish commonly targeted by recreational anglers. The publication first highlights how artificial reefs potentially affect some ecological processes, and then it describes how these changes may translate into overall population-level effects for some species. This fact sheet should help the interested public understand more about the ecological effects of artificial reefs, as well as providing detailed information to key stakeholders including management agencies, local government personnel, artificial reef manufacturers, and Extension agents. Specifically, it will provide a greater understanding of how artificial reefs affect fish, and this information should allow for more informed decisions about building and managing artificial reefs.
How do artificial reefs affect fish? Some Terminology
Artificial reefs affect fish populations in direct and indirect ways that can alter populations in different ways. In this fact sheet we describe “direct effects, “indirect effects,” and "total effects.” Direct effects are those that affect fish behaviors immediately. Some examples would be changes in how fish feed, hide, or move. “Indirect effects” are the differences in vital rates that happen as a result of those fish behavior changes brought on by the direct effects of the artificial reefs. (Vital rates are population demographics like survival, reproduction, death, growth, etc.) Finally, “total effects” are overall fish population changes, for example, greater or lesser abundance or increased or decreased movement.
Direct Effects
The two direct ways artificial reefs can benefit fish are by providing them more structure for shelter and by increasing their forage. Shelter is habitat fish can use to conceal themselves from hunting predators. Forage is feeding opportunities.
The physical structure of artificial reefs (or what grows on them) provides fish with shelter and refuge (or hiding places). Shelter is often thought to be important for smaller species or younger individuals, since smaller fish have more predators. However, larger fish like grouper also need shelter from even larger predators like sharks.
The other main way artificial reefs benefit fish is by providing foraging opportunities. Artificial reefs provide good growing surfaces for the algae and sessile invertebrates that provide forage for other smaller organisms, like sea urchins, crabs, and smaller crustaceans (Pickering and Whitmarsh 1997). These reefs may attract baitfish (e.g., scad, sardines, menhaden, etc.). In turn, these baitfish can attract larger species looking for foraging themselves (McGlennon and Branden 1994). Some of the larger species may become residents of the reef, taking shelter and often ambushing prey around the structure the reefs provide. Common examples of fish that do this include grunts, snappers, and groupers. Other more mobile predators may visit the reefs, either looking for opportunities to attack smaller baitfish, or potentially even eating the large fish sheltering and foraging around the reefs. Examples of these predators might include sharks, mackerels, and members of the jack family.
In short, fish can benefit from the shelter artificial reefs provide for refuge, or the foraging opportunities provided by the other fish and animals that occupy or have colonized the reef. It is important to remember that most species of fish are both predators and prey. This means artificial reefs may improve foraging opportunities for medium-sized fish like white grunt, but at the same time they may also provide larger predators like grouper and sharks good opportunities to feed on these same white grunt. The net effect on the local fish populations (for example, white grunt) would then depend on the improvement in the grunt’s foraging, as well as any improvement in the foraging of the grunt’s predators.
Indirect Effects
It seems obvious that direct effects of increased refuge or foraging opportunities would translate to changes in vital rates like body growth or survival. More good hiding places (refuge) should translate to better survival (fewer fish being eaten by predators). And fish that have lower mortality (survive better) should, in general, produce more eggs. Similarly, more foraging opportunities should allow greater growth. Fish that grow faster get bigger faster, and at larger sizes will have less natural mortality (better survival) and be more likely to produce more eggs. But also, research has shown that fish are most vulnerable to predation when they themselves are foraging (van Poorten et al. 2018; Camp et al. 2019). This suggests that “better forage” opportunities may benefit fish in two ways. It may not only be that fish have access to more food, but also that the time they spend foraging—and with it their risk—will be reduced. In short, decreased forage time due to easier access to food should reduce both energy expenses and predation risk, thus increasing survival rates (Ahrens et al. 2012). Better forage opportunities may directly decrease fish mortality rate, allowing them to survive longer and produce more eggs. The main point is that either better forage or refuge from artificial reefs should cause fish to survive better and allow more larger fish that will spawn and create more eggs. This is true as long as other vital rates do not change.
What makes the indirect effects more complicated to understand is that often other things do change. We can use the white grunt example again. If artificial reefs provide better foraging for grunt without changing the rate at which grunt are predated on by larger fish, it is clear there will be a benefit to the grunt in terms of more large fish to spawn. But what if the grunts increased foraging, but larger grouper also increased foraging on the grunts? Then the indirect effects on grunt survival become more difficult to assume and would depend on which effect was greater. It is important to recognize that while many fish species may use artificial reefs, how much they depend on them may vary quite a bit. This could cause the effect the artificial reefs have on fish vital rates like growth and survival. For example, compared to “resident” species that may spend much of their lives at the same reef site like vermillion or lane snapper (Allman 2007), other species like red drum, cobia, jacks, barracudas, etc. may visit reefs (Paxton et al. 2020). What this means is that the net effects of artificial reefs on a local population depend not only on how the reef affects a given species of fish, but also how the reef affects that species’ prey and predators.
Artificial reefs may affect survival of fish at just about any life stage, but the population benefits are likely to be most noticeable if artificial reefs affect survival during the life stage referred to as the “recruitment” period. “Recruitment” is simply the survival of small fish long enough to become larger fish and join sub-adult or adult populations. During the recruitment period, the mortality of the fish is “density dependent.” Density-dependent mortality means the mortality depends on how many fish there are in an area. More small fish means a lower survival rate. This is because small fish strongly compete for resources like forage and especially shelter. In FA222 and FA234, recruitment is described in greater detail. It has been difficult to assess exactly when recruitment processes occur, but they probably start shortly after settlement and persist until somewhere between 10–20% of the average adult size (Lorenzen and Camp 2019).
Recruitment to an artificial reef is from larval fish (or movements of post-settlement fish) to reefs that contribute to the adult fish population (Victor 1986). The hard complex structures of artificial reefs attract larval fish from the pelagic water and offer habitat for them when they settle. Artificial reefs can be put in places with bare bottom or no natural reefs and allow larval fish to settle that otherwise would have been lost to the environment (Carr and Hixon 1997). These structures then allow for greater recruitment of certain species because they can provide additional shelter areas and/or forage opportunities for settled fish that will decrease competition for hiding spaces and food (Caddy 2011; Bortone 2019). This will allow more fish to survive through this recruitment period when mortality is density dependent. However, if artificial reefs do attract more predators, especially in crowded areas, larval fish of the prey species will likely compete for space and food. Therefore, species that may settle on natural or artificial reefs structures are more likely to have their recruitment affected by artificial reefs than species who do not display an affinity for structure (migratory species like jacks and barracuda).
There has been relatively little research clearly showing which species of fish actually recruit (survive the period of density-dependent mortality) on artificial reefs. There is some evidence that some snappers (e.g., red snapper and lane snapper), as well as grunt (e.g., white grunt) can recruit to artificial reefs (Beets 1989; Bohnsack et al. 1994; Gallaway et al. 2009; Brandt and Jackson 2013; Patranella et al. 2017). However, in several of these studies the artificial reefs used were designed and placed as part of scientific experiments, often in shallower waters, and so we cannot assume these fish regularly recruit to the same types of artificial reefs fishers often imagine visiting. Also, there is more evidence suggesting many of these more popular sport fish may also settle in habitat closer to shore, like sea grass, low-relief shell banks, or mud flats (Dance et al. 2021). For example, certain species such as mangrove snapper are understood to recruit in estuarine areas but may occupy natural and artificial reefs as they mature (Flaherty-Walia et al. 2015). More research is needed to understand what specific type of artificial reef structures, design, and construction create more habitat for recruitment, especially of desired sportfish. The direct and indirect effects of artificial reefs are summarized in Figure 1.
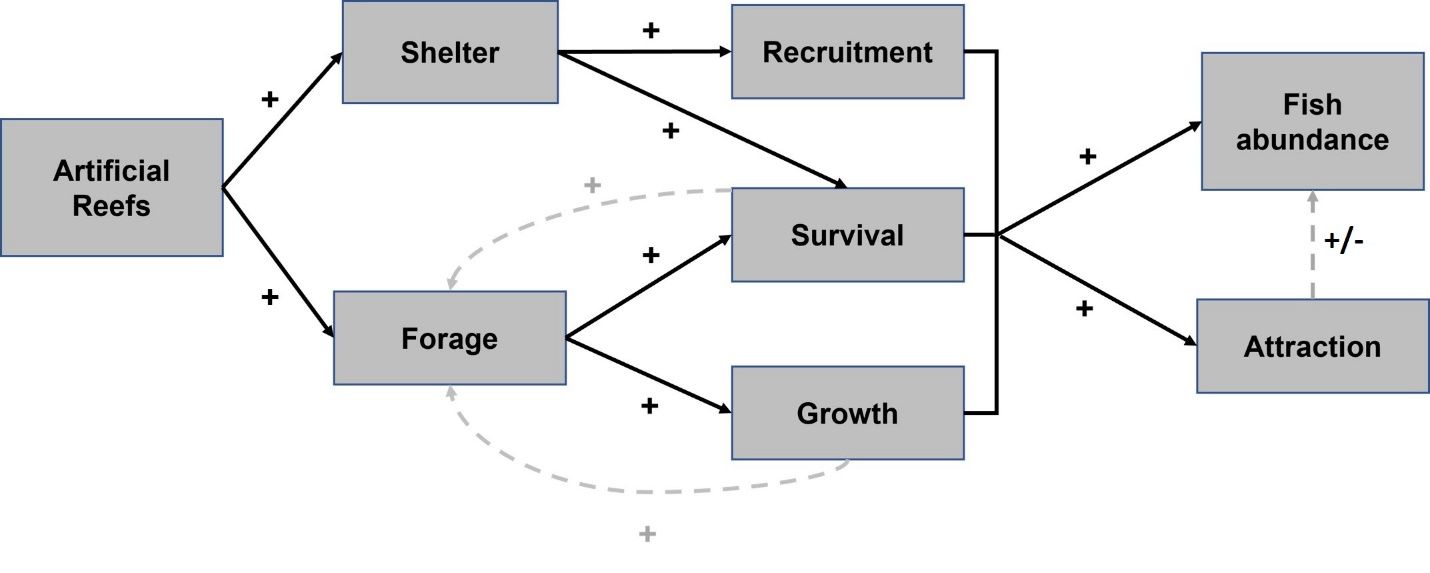
Credit: Lisa Chong, UF/IFAS
Total Effects on Fish Populations
The total effects of artificial reefs on fish populations will depend on how survival across different fish species is impacted and whether the reproductive output (number of eggs) increases. This impact is illustrated with a simple example by imagining the population of a single species, like the white grunt, and assuming this species but not its predators are affected by artificial reefs. If the artificial reef provides adult white grunt with more forage habitat, this will lead to greater growth and survival, and ultimately to greater abundance of adult fish. More fish would live longer and produce more young fish. Alternatively, if the artificial reef provides recruitment habitat for juvenile white grunt, more would “recruit” to the sub-adult and adult population. In either case, the artificial reef would increase the total amount of adult white grunt in the region. But the artificial reef might also change how these fish are distributed through the region—by attracting them. Fish could be attracted because we think fish are good at finding the places where they have the best (or at least good) forage. That means that if the artificial reef was providing better forage than surrounding sites, it could also be attracting white grunt from other areas. This attraction would increase the number of white grunt on that artificial reef, and would increase the density of white grunt at artificial reefs.
This situation leads to two separate, somewhat complicated phenomena. First, it becomes difficult to assess if fish at artificial reefs have been “attracted” from other areas, or actually recruited and “produced” by the reef. Scientists have debated this “attraction vs. production” issue for decades (Pickering and Whitmarsh 1997; Lindberg et al. 2006; Caddy 2011). It is probably not a matter of which occurs, but which is the greater force because both are likely to occur (Lindberg and Seaman 2011). Whether attraction or production is greater will probably depend on specific situations, including the species, types of artificial reefs, and habitat quality. In general, if artificial reefs are attracting fish more than producing them (via recruitment), we think they probably have a lesser effect on the local fish population (across natural and artificial areas). However, the fact that fish are attracted at all would seem to suggest there is some benefit to them (such as increased growth or decreased mortality) (Lindberg et al. 2006). This leads to the second complicating factor, which is density dependence. As more fish are attracted to an artificial reef, their density increases. Increasing density usually results in decreased growth rates, which in turn affects mortality, which then affects density. So what does all this mean? If we were to think about the effects of artificial reefs on a single species of fish that uses the reef, and assume its predators did not change, artificial reefs are likely to have some positive effect. However, that effect is likely lesser if the fish are attracted from another area, as opposed to “produced” in some way by the reef. Further, these positive effects may be somewhat diminished by changes in density of fish.
However, these hypothetical examples are unrealistic in that they do not consider any predators of the fish. Predators (in this hypothetical example, sharks) might also be attracted and could themselves use the reef to forage (Hixon and Beets 1989; Lindberg et al. 2006). This could diminish some of the population gains from the greater shelter and forage. In extreme cases, it could even lead to an ecological trap. An ecological trap is a scenario when some environmental cue leads organisms to settle on poor-quality habitats. In our example with white grunt, an ecological trap could occur if artificial reefs attract not only white grunt but their predators like sharks or larger fish, and if for whatever reasons the white grunt are easier for their predators to catch around artificial reefs. In this ecological trap, the white grunt might keep getting attracted to the reef but would then suffer greater mortality there. However, we do not think this is likely to occur very often, at least with most natural predators. But it is worth considering that a similar thing could occur if the predator was a recreational fisher. This effect will be described in part three of the series (FA243). In all, it is reasonably safe to assume artificial reefs result in some additional benefit to fish populations, at least if one does not consider how artificial reefs may affect fishing harvest rates.

Credit: Michael Dickson, UF/IFAS
Summary
How artificial reefs affect fish depends on how well they can shelter fish and provide fish with the resources they need for growth and survival. Most of the time, artificial reefs by themselves should have some positive effects on fish populations. Artificial reefs would increase fish populations most by providing good habitat for recruitment of small fish. The design and specific attributes of artificial reefs can also determine how well they shelter fish and provide them with forage. This is ongoing research and probably depends on location and type of species. Attraction is not necessarily a bad thing, but if it leads to ecological traps (either attracting larger predators or both fish and fishers at high rates), then artificial reefs can become problematic for fish populations. Because fishing is a common activity at artificial reefs, and because the number of fishers on the water continues to increase with population growth, we must consider how fishers interact with artificial reefs and how fishing affects fish populations. We discuss how artificial reefs affect fishers in part three of this series (FA243).
References
Ahrens, R. N., C. J. Walters, and V. Christensen. 2012. “Foraging Arena Theory.” Fish and fisheries 13 (1): 41–59.
Allman, R. J. 2007. “Small-Scale Spatial Variation in the Population Structure of Vermilion Snapper (Rhomboplites aurorubens) from the Northeast Gulf of Mexico.” Fisheries Research 88 (1–3): 88–99.
Beets, J. 1989. “Experimental Evaluation of Fish Recruitment to Combinations of Fish Aggregating Devices and Benthic Artificial Reefs.” Bulletin of Marine Science 44 (2): 973–983.
Bohnsack, J. A., D. E. Harper, D. B. McClellan, and M. Hulsbeck. 1994. “Effects of Reef Size on Colonization and Assemblage Structure of Fishes at Artificial Reefs off Southeastern Florida, USA.” Bulletin of Marine Science 55 (2–3): 796–823.
Bortone, S. A. 2019. “Artificial Reefs in the Future Management of Red Snapper, Lutjanus campechanus.” In Red Snapper Biology in a Changing World (pp. 275–283). CRC Press.
Brandt, J. R., and D. C. Jackson. 2013. “Influences of Artificial Reefs on Juvenile Red Snapper along the Mississippi Gulf Coast.” Marine and Coastal Fisheries 5 (1): 1–10.
Caddy, J. F. 2011. “How Artificial Reefs Could Reduce the Impacts of Bottlenecks in Reef Fish Productivity within Natural Fractal Habitats.” In Artificial Reefs in Fisheries Management, edited by S. A. Bortone, F. P. Brandini, G. Fabi, and S. Otake 45–64. Boca Raton, FL: CRC Press.
Camp, E. V., R. N. Ahrens, T. C. MacDonald, K. A. Thompson, and K. Lorenzen. 2019. “Identifying Forage Populations of Concern: A New Perspective Based on Predator Recruitment Considerations.” Fisheries Research 219:105319. https://doi.org/10.1016/j.fishres.2019.105319
Carr, M. H., and M. A. Hixon. 1997. “Artificial Reefs: The Importance of Comparisons with Natural Reefs.” Fisheries 22 (4): 28–33.
Dance, M. A., W. F. Patterson III, and D. T. Addis. 2011. “Fish Community and Trophic Structure at Artificial Reef Sites in the Northeastern Gulf of Mexico.” Bulletin of Marine Science 87 (3): 301–324. https://doi.org/10.5343/bms.2010.1040
Dance, M. A., J. R. Rooker, R. J. Kline, A. Quigg, G. R. Stunz, R. D. Wells, K. Lara, J. Lee, and B. Suarez. 2021. “Importance of Low‐Relief Nursery Habitat for Reef Fishes.” Ecosphere 12 (6): e03542.
Flaherty-Walia, K. E., T. S. Switzer, B. L. Winner, A. J. Tyler-Jedlund, and S. F. Keenan. 2015. “Improved Ability to Characterize Recruitment of Gray Snapper in Three Florida Estuaries along the Gulf of Mexico through Targeted Sampling of Polyhaline Seagrass Beds.” Transactions of the American Fisheries Society 144 (5): 911–926.
Gallaway, B. J., S. T. Szedlmayer, and W. J. Gazey. 2009. “A Life History Review for Red Snapper in the Gulf of Mexico with an Evaluation of the Importance of Offshore Petroleum Platforms and other Artificial Reefs.” Reviews in Fisheries Science 17 (1): 48–67.
Hixon, M. A., and J. P. Beets. 1989. “Shelter Characteristics and Caribbean Fish Assemblages: Experiments with Artificial Reefs.” Bulletin of Marine Science. 44 (2): 666–80.
Lindberg, W. J. 1997. “Can science resolve the attraction-production issue?” Fisheries 22: 10–13.
Lindberg, W. J., T. K. Frazer, K. M. Portier, F. Vose, J. Loftin, D. J. Murie, D. M. Mason, B. Nagy, and M. K. Hart. 2006. “Density‐Dependent Habitat Selection and Performance by a Large Mobile Reef Fish.” Ecological Applications 16 (2): 731–46. https://doi.org/10.1890/1051-0761(2006)016[0731:DHSAPB]2.0.CO;2
Lindberg, W. J. and W. Seaman. (editors). 2011. Guidelines and Management Practices for Artificial Reef Siting, Use, Construction, and Anchoring in Southeast Florida. Florida Department of Environmental Protection. Miami, FL. xi and 150 pages.
McGlennon, D. and K. L. Branden. 1994. “Comparison of Catch and Recreational Anglers Fishing on Artificial Reefs and Natural Seabed in Gulf St. Vincent, South Australia.” Bulletin of Marine Science 55 (2–3): 510–523.
Patranella, A., K. Kilfoyle, S. Pioch, and R. E. Spieler. 2017. “Artificial Reefs as Juvenile Fish Habitat in a Marina.” Journal of Coastal Research 33 (6): 1341–1351.
Paxton, A. B., E. A. Newton, A. M. Adler, R. V. Van Hoeck, E. S. Iversen Jr., J. C. Taylor, C. H. Peterson, and B. R. Silliman. 2020. “Artificial Habitats Host Elevated Densities of Large Reef-Associated Predators.” PloS one 15 (9): e0237374.
Pickering, H., and D. Whitmarsh. 1997. “Artificial Reefs and Fisheries Exploitation: A Review of the ‘Attraction versus Production’ Debate, the Influence of Design and its Significance for Policy.” Fisheries Research 31 (1–2): 39–59. https://doi.org/10.1016/S0165-7836(97)00019-2
Van Poorten, B., J. Korman, and C. Walters. 2018. “Revisiting Beverton–Holt Recruitment in the Presence of Variation in Food Availability.” Reviews in Fish Biology and Fisheries 28 (3): 607–624. https://doi.org/10.1007/s11160-018-9521-6
Victor, B. C. 1986. “Larval Settlement and Juvenile Mortality in a Recruitment‐Limited Coral Reef Fish Population.” Ecological Monographs 56 (2):145–160.
Wilson, J., C. W. Osenberg, C. M. S. Mary, C. A. Watson, and W. J. Lindberg. 2001. “Artificial Reefs, the Attraction-Production Issue, and Density Dependence in Marine Ornamental Fishes.” Aquarium Sciences and Conservation 3 (1): 95–105. https://doi.org/10.1023/A:1011343312031