Summary
Florida’s economically important estuaries could be heavily impacted by sea-level rise and altered river flow, both caused by climate change. The resulting higher salinity, or saltiness of the water, could harm plants and animals, alter fish and bird habitat, and reduce the capacity of estuaries to provide such important services as seafood production and the protection of shorelines from erosion. This publication contains information for stakeholders, students, scientists, and environmental agencies interested in understanding how changes in salinity impact Florida’s estuaries with specific case studies.
Introduction
Estuaries are one of the most productive kinds of ecosystems on Earth, and they support a high diversity of plants, fish, birds, and other animals. Estuaries are valuable for biodiversity and also have a high economic value for Florida. Many commercially and recreationally fished species caught off Florida’s coasts, including shrimp, crabs, oysters, clams, lobsters, red drum, common snook, spotted seatrout, and certain groupers and snappers, depend on estuaries for at least part of their life cycles. More information about how estuaries affect juvenile fish can be found in EDIS publications #FA250, “How Oyster Reefs Can Affect Finfish Recruitment,” and #FA239, “Ecological Influences on Coastal Finfish Recruitment.” Marine fisheries depend on mangrove, marsh, and seagrass habitats in estuaries for nursery habitat where the density, growth, and survival of juveniles is higher than other areas (Lefcheck et al. 2019; Love et al. 2022a, 2022b). In addition, the seagrasses and mangroves around estuaries offer habitat for millions of birds and other animals while providing critical human services like stabilizing shorelines and cleaning water (Smyth et al. 2022). These are just a few of the many benefits that coastal habitats like estuaries provide to Florida’s people (Wallace et al. 2023). One of the main reasons these areas are so valuable is because they are where fresh and saltwater meet.
Estuaries are bodies of water along the coastline that can be relatively enclosed bays or wide marshes at river mouths. They are places where fresh water from rivers mixes with saltwater from the sea, creating a place with intermediate salinity. On average, the salinity of the open ocean is 35 parts per thousand (ppt). The salinity of rivers can range from 0.1 to 5 ppt. In estuaries, salinity is highly variable because of tidal effects and because of variation in freshwater inflow from rivers (Figure 1).
When freshwater inputs are low, an estuary can become as salty as the adjacent ocean. When freshwater inputs are high, an estuary can become entirely fresh. Even during normal river flow, salinity in an estuary varies between high and low tide. The salinity is higher during high tide because more ocean water is entering the estuary. Salinity is lower at low tide because fresh water is moving in as the ocean level is receding.

Credit: Adapted from Peter Summerlin, Louisiana State University; CC BY-SA 3.0
The organisms that live in estuaries (Figure 2 and Figure 3) are adapted to the variations in salinity. Typically, there is a gradient, from species that prefer fresher water at the upstream river end of the estuary to species that prefer saltier water at the downstream ocean end.
The freshwater plant Vallisneria, sometimes called wild celery or “eelgrass or tape grass,” occurs at the upper end of estuaries and in rivers, and it prefers salinities in the range of 0 to about 5 ppt. It can tolerate salinities up to 18 ppt, but only for short periods of time (Doering, Chamberlain, and McMunigal 2001). Further toward the ocean, plants such as Thallasia, a type of seagrass commonly called turtle grass, prefer salinities in the 30 to 40 ppt range but can tolerate lower salinities for short periods of time, such as when there is a large discharge of fresh water from a river into the estuary (Lirman and Cropper 2003). Crassostrea virginica, the eastern oyster, has a preferred salinity range of 14 to 28 ppt (Shumway 1996), but it can tolerate short exposures to fresh water or ocean water.
Plants and animals in estuaries are harmed when unusually high or low salinities persist for prolonged periods of time. The higher salinity, reduced nutrient input, and reduced sediment inputs associated with extreme low river inflow can stress or kill plants and animals; diminish productivity due to lack of nutrients; and kill marshes due to reduced sediment input. For plants, high salinity can also cause osmotic stress. But long periods of high freshwater inputs from rivers are also problematic. With extreme high river inflow, low estuary salinity kills marine organisms; high nutrient inputs contribute to algal blooms; and increased sediment input smothers sea grasses and oysters. In both cases, extreme events are the issue.

Credit: Laura K. Reynolds, UF/IFAS

Credit: UF/IFAS
Examples of Stress from Extreme Levels of Salinity
The following are examples of harm caused to estuaries due both to periods of high salinity from extreme low freshwater input and to periods of low salinity from extreme high freshwater input.
Florida Bay
Florida Bay is a shallow, triangle-shaped estuary at the southern tip of Florida. Freshwater inputs occur primarily through rainfall and sheet flow from the Everglades which can vary by season, weather, and management action. Because the bay is divided up into basins that are only connected by channels, salinity within the bay can vary significantly (Rudnick et al. 1999). In the late summer of 1987, there were large reports of seagrass die-off in the north central part of Florida Bay (Zieman et al. 1999). The cause of that die-off is debated, but the dominant hypothesis is that high temperatures and high salinities increased plant oxygen demand. As the plants consumed available oxygen, there was less oxygen available in the sediment and bottom water, causing sulfide to build up, which is toxic to seagrasses. As plants died and decomposed, oxygen became even more limiting and toxic sulfide more prevalent (Carlson, Yarbro, and Barber 1994). As seagrasses die, they no longer hold sediment in place and decomposition of the plant material releases nutrients that were bound in plant tissue. Widespread turbid, cloudy water and recurrent algal blooms led to secondary seagrass loss and a cascade of ecological change including sponge mortality and reductions in fisheries (Durako, Hall, and Merello 2001; Peterson et al. 2006). These conditions slowly improved over about a decade (Durako, Hall, and Merello 2001). However, in 2015, researchers began reporting a reoccurrence—elevated salinities, high sulfide levels, seagrass die-off, and fish kills (Hall et al. 2016). Increasing freshwater flow to Florida Bay through alterations in water management is a common suggestion for reducing the likelihood of more die-off events in the future.
Apalachicola Bay
In the fall of 2012, Apalachicola Bay, located in the Big Bend region of Florida next to the northeast corner of the Gulf of Mexico, experienced a rapid decline in its oyster population that has not recovered, as of 2022 (Johnson, Pine, and Camp 2022). It coincided with salinities as high as the adjacent ocean, caused by two successive years of record low river inflows, and unprecedented high fishing effort and harvest (Camp et al. 2015). It also coincided with high levels of oyster predators, such as crabs and conchs; parasites, such as worms, snails, and sponges (Figure 4); and pathogens, such as Perkinsus, a group of parasitic protists (single-celled organisms) that infect certain marine animals. Research showed the oyster population collapse was likely related to decreased survival of juvenile oysters (Pine et al. 2015; Johnson et al. 2023). Additionally, results from field experiments demonstrate that as the salinity in Apalachicola increased, so did the presence of an oyster predator called oyster drills (Kimbro et al. 2017). It is possible that this lower survival of juvenile oyster was made worse by high salinity or other environmental conditions (Camp et al. 2015; Kimbro et al. 2017). However, links between salinity and oysters are not clear (Fisch and Pine 2016), and juvenile survival appears to have remained low in the 2012–2022 period despite variable and more normal water conditions (Johnson et al. 2023). Increasingly it seems that the oyster population in Apalachicola Bay experienced a shift to a much lower (and less valuable) population level (Johnson, Pine, and Camp 2022). Ultimately it is not known what role high salinity may have played in that initial population shift (Johnson et al. 2023), but it is possible it played one since salinity and temperature are key environmental factors that structure oyster populations (Love et al. 2021).
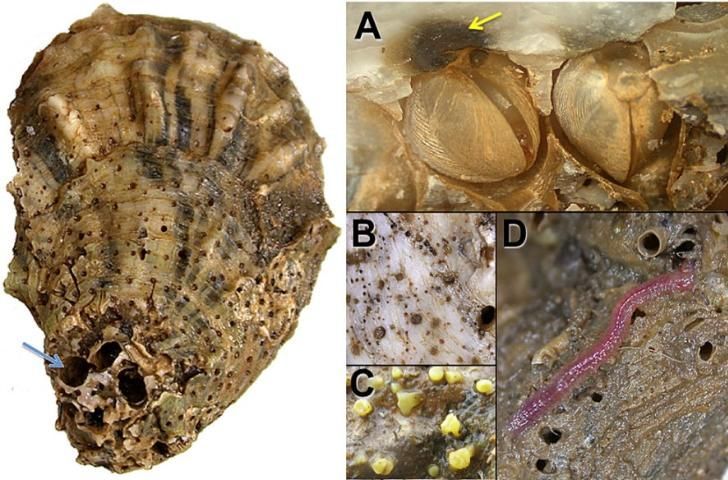
Credit: Andrew Kane, University of Florida
An earlier decline in oysters in Apalachicola Bay was documented by Petes, Brown, and Knight (2012). They also found that during a time of reduced freshwater inflows and elevated salinities, oysters experienced mortality. They attributed the deaths to Perkinsus and suggested that the high salinity enhanced the growth of Perkinsus populations. The study also identified a situation seen in other research: a synergistic negative effect of high salinity and high water temperature on oyster health.
Biscayne Bay
Biscayne Bay is a subtropical, low-nutrient estuary in southeast Florida. Urban growth in north Biscayne Bay, agricultural activities in south Biscayne Bay, and water management practices such as canal drainage have led to a decline in water quality in the region, including changes in salinity and nutrients. Salinity in Biscayne Bay has been slowly increasing and moving from estuarine to more marine (Wingard et al. 2004) due to decreased freshwater flow from natural rivers and decreased sheet flow from the Everglades. Canals now bring freshwater and nutrients into Biscayne Bay (Cary et al. 2011; Caccia and Boyer 2007). The increased delivery of nutrients with freshwater has led to algal blooms and periods of low oxygen in Biscayne Bay, which are associated with die-offs of seagrass beds in the region (Millette et al. 2019). These fluctuations in salinity due to freshwater input from canals may also affect the size and distribution of seagrasses. Model simulations suggest that a decrease in salinity will shift the dominant seagrass species, which has consequences for fisheries and other ecosystem services (Lirman and Cropper 2003).
Seagrasses are not the only marine species affected by the change in salinity in Biscayne Bay. There are historical reports of a healthy eastern oyster population (Harlem 1979; Meeder, Harlem, and Renshaw 2001). Past fisheries data show more frequent catches of estuarine species, indicating more brackish conditions (Serafy et al. 1997). Yet, long-term reduction in freshwater discharge associated with canalization of Miami, have led to reduced freshwater flow and an increase in salinities. Oysters have a very specific salinity tolerance, so salinity changes to their environment can have consequences for the oyster populations. In Biscayne Bay, the high salinity events have been linked to the loss of reef-building oysters (Parker et al. 2013). Oysters that remain in Biscayne Bay are smaller. Though live oysters indicate a reproducing population of oysters, the lack of larger oysters indicates high mortality, likely associated with high salinity (Meeder, Harlem, and Renshaw 2001). Unfortunately, changes in freshwater flow to create a more brackish environment favorable for oysters will not restore the oyster population in Biscayne Bay, as hard substrate and other places for oysters to settle and grow is lacking (Parker et al. 2013).
St. Lucy Estuary
The St. Lucie Estuary, located along the Atlantic coast of Florida, periodically receives large discharges of fresh water from Lake Okeechobee via a canal that was built between the lake and estuary in the 1960s. When this happens, a variety of negative effects occur (Sime 2005), including reduced light availability due to material in the water, which causes loss of submerged plants, as well as impacts to oysters, coral reefs, and benthic animal diversity.
Freshwater inputs also bring large amounts of nutrients. Usually these do not stimulate blooms of algae because the water is moving too fast for them to proliferate (Phlips et al. 2012) and nutrients are quickly diluted in the larger estuary, but under certain conditions, blooms have occurred. Increased nutrients in estuaries can also lead to the decrease of seagrass beds and an increase in proliferations of benthic macroalgae, which can be problematic by emitting noxious odors, decreasing dissolved oxygen when decomposing, harboring pathogenic microorganisms, and producing toxins by benthic cyanobacteria (Ford et al. 2018; Ford et al. 2021; Wood et al. 2020; Berthold, Lefler, and Laughinghouse 2021; Lefler, Berthold, and Laughinghouse 2021).
Projected Changes in Climate That Could Affect Salinity in Estuaries
Climate change is expected to cause two major responses that will directly affect the salinity of estuaries: (1) altered river flow and (2) a continued rise in sea level. The Intergovernmental Panel on Climate Change (IPCC 2019) projects that global average sea level will rise between 2 and 3.6 feet during this century, and that it is “virtually certain” that sea level will continue to rise after 2100. If global warming passes a certain threshold, projected to be between 2°F and 3°F, there could be near-complete loss of the Greenland Ice Sheet, causing a global mean sea-level rise of about 20 feet.
While sea levels are rising, climate change is expected to influence the pattern of rainfall and drought. Wuebbles et al. (2014) ran 40 climate models and found that they all gave common predictions for the future: warming of the atmosphere and soil, lower relative humidity across the United States, and an increase in the duration, frequency, and intensity of droughts. Murray, Foster, and Prentice (2012) ran several climate models, and they all predicted that climate change and population growth will result in increased global water stress in this century. Sheffield and Wood (2008) projected that there will be a doubling in the frequency of droughts lasting four to six months and a three-fold increase in the frequency of droughts lasting longer than a year.
What do these changes—altered river flow and continuing sea-level rise—mean for estuaries and their salinity? Regarding river flow, there will be prolonged periods of low flow during droughts and then short periods of extreme high flow. Because the interaction of the ocean and river water is what creates the intermediate salinity of estuaries, the expected outcome is long periods of higher estuarine salinity interrupted for short periods with a flush of fresh water. Higher sea level will exacerbate the high salinity during low-flow periods (Figure 5). Sea-level rise will not only increase salinity; it will also increase submergence times, meaning areas will be under water for longer periods of time.
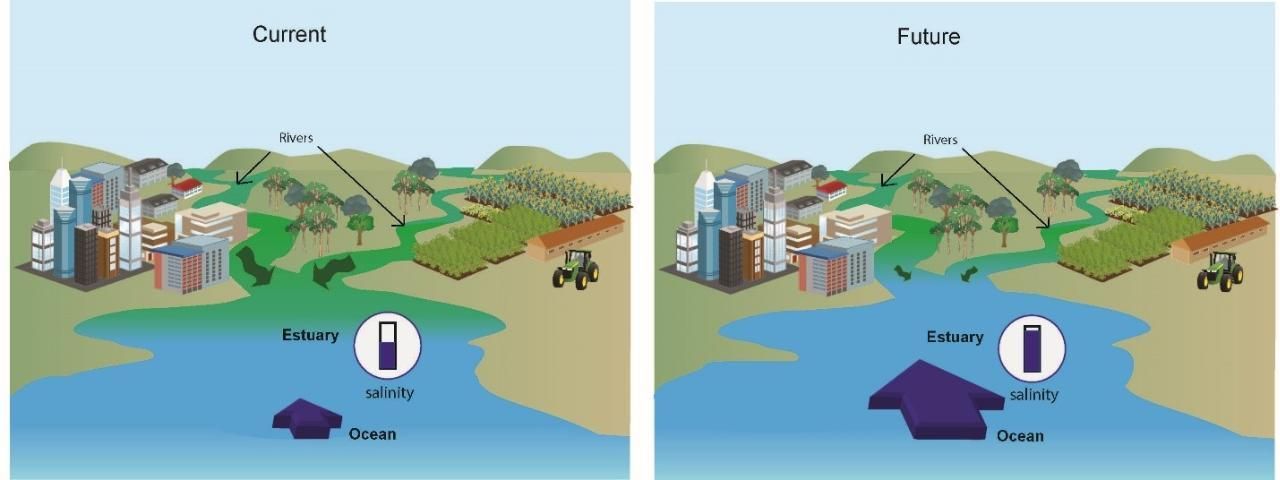
Credit: Florida Sea Grant
How Changes May Affect Estuarine Plants and Animals
A future in which sea-level rise causes saltier ocean water to move into estuaries and longer-lasting droughts that usher in periods of reduced freshwater input, combined with longer submergence times, could stress estuarine organisms. The range of salinity may exceed what is optimal for their growth and even exceed what they can tolerate (Figure 6). Species that cannot swim might be greatly reduced in numbers. Others might be able to migrate further upstream into the rivers that feed the estuary to reach a place where they can tolerate the salinity. Sea-level rise may also lead to more and different pathogens, pests, and predators that will affect the health and survival of estuarine plants and animals.
One problem is that most of Florida’s estuaries, with the exception of bays fed by sheet flow through the Everglades, are fed by rivers that are much narrower than the estuary and are often dredged and kept deeper for boat traffic. This means there is much less space in rivers for estuary organisms to use if the estuary or bay becomes saltier than they prefer. Additionally, the movement of species from estuarine areas that become too salty requires there to be usable passageways to upstream river areas and usable habitat when they get there. These requirements are often not met since many rivers have barriers to movement (dams, culverts, or other water control structures) and also often feature developed or hardened shorelines (e.g., with retaining walls or bulkheads; Wallace, Camp, and Smyth 2022). The term “coastal squeeze” is used to describe this phenomenon when human structures prevent the landward migration of coastal species and squeeze out their habitats.
Sea-level rise and associated changes in salinity and in the inundation of coastal habitats have also been linked to peat collapse in marshes of the Everglades (Wilson et al. 2018; Chambers, Steinmuller, and Breithaupt 2019). Peat is the partially decomposed organic material found in coastal wetlands and is the base on which wetland plants, like mangroves and sawgrass, grow. As sea levels rise and these areas become wetter and saltier for longer periods of time, plants become stressed and die. As a result, ecosystem processes that are normally slow speed up. One example of this is the process of decomposition. As this happens, that peat no longer builds up but rather collapses, leaving holes in the wetland prairie. In this case, the increase in salinity causes the plants to die. With the loss of plants to stabilize the peat, coupled with the increase in decomposition, the peat will eventually collapse.
There is a rich scientific literature on the relationship between freshwater inflow and subsequent commercial landings of fish and shellfish. Some research suggests the increase of salinities will negatively affect fish populations and catch (Jenkins, Conron, and Morison 2010). Other research suggests the lowering of salinity will not increase landings of species such as oysters (Turner 2006), and some found no consistent trends between freshwater flow and landings (Fisch and Pine 2016). In Florida (Green et al. 2006) and further worldwide (Nordlie 2003; Feyrer et al. 2015), changes in estuarine salinity conditions will likely have some effect on fish communities. The specific changes will depend on the species and the estuary, but generally more extreme patterns (high and low) in salinity will be problematic for many species and the fisheries that depend on them.
If the salinity of places like Apalachicola Bay and Tampa Bay increases into the 35 ppt range, ocean-dwelling predators, parasites, and pathogens will be able to thrive in the estuaries and will likely have some effect on the ecosystems. Vallisneria americana (wild celery) and other low-salinity-adapted species of aquatic plants at the upper end of the estuary may be constrained to a smaller space or may not be able to survive. Marine macroalgae or seagrasses could overtake these habitats and affect the estuary food web, impacting productivity and fisheries due to changes in food availability. These changes in the primary producer community will affect the productivity of economically important fisheries, ultimately making estuaries very different than what we see today.
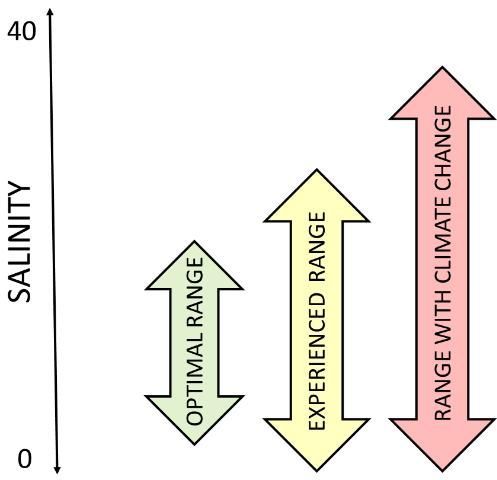
Credit: Florida Sea Grant
Water Use and Consumption
One of the big unknowns that will affect river flow into estuaries is the use of fresh water by people. It is critical now and especially in a future with increased droughts and saltwater intrusion that water be used in as conservative a manner as possible.
Florida’s water management districts have protocols and plans to minimize wasteful use of fresh water, and they need to be followed. The average person in Florida may think that we are a state with plentiful water. Yet, the reality is that our aquifers are being drawn down (i.e., lowering of the water table by groundwater withdrawal), leading to dry wells. At the same time, as sea levels rise, saltwater moves inland leading to saltwater intrusion. Saltwater can easily spread through groundwater. Saltwater is denser than fresh water and forms a wedge under the fresh water. As we draw down our aquifers and wells, this new space is replaced by saltwater. In Hallandale Beach (Broward County), saltwater has breached five of its eight freshwater drinking wells.
We also have networks of coastal canals that quickly discharge water to the ocean during the rainy season to protect our buildings and crops from flooding. That water is essentially wasted because it is no longer available in the dry season. This also affects coastal ecosystems because when water is quickly discharged in the rainy season, it causes harm to salt-tolerant species. In the dry season, there often is not enough freshwater flow to sustain species that cannot tolerate high salinity.
This management scheme needs to change and may require costly water storage facilities to capture some or all of that water for later use when rainfall is scarce.
Other Effects of Climate Change on Estuaries
This publication focused on effects of climate change resulting from altered salinity in estuaries. It did not consider some of the other substantive changes that may occur and that will be addressed in other publications.
Those changes include tropicalization. Tropicalization is the movement of tropical and subtropical plants and animals north because of increases in temperature. Another change that may occur because of warming water is the intensification of toxic algal blooms and of disease-causing microorganisms. Increased temperature stimulates faster growth of those organisms and their populations—with potential negative effects on fish, oysters, clams, and people who recreate in or live near the water. This particular topic is covered in "Climate Change and the Occurrence of Harmful Microorganisms in Florida's Ocean and Coastal Waters" (https://doi.org/10.32473/edis-sg136-2015), available on EDIS by Havens (2015).
For additional general information on estuaries, see the online lessons from NOAA: http://oceanservice.noaa.gov/education/tutorial_estuaries/welcome.html
Literature Cited
Berthold, D. E., F. W. Lefler, and H. D. Laughinghouse IV. 2021. "Untangling Filamentous Marine Cyanobacterial Diversity from the Coast of South Florida with the Description of Vermifilaceae Fam. Nov. and Three New Genera: Leptochromothrix gen. nov., Ophiophycus gen. nov., and Vermifilum gen. nov." Molecular Phylogenics and Evolution 160: 107010. https://doi.org/10.1016/j.ympev.2020.107010
Caccia, V. G., and J. N. Boyer. 2007. “A Nutrient Loading Budget for Biscayne Bay, Florida.” Marine Pollution Bulletin 54 (7): 994–1008. https://doi.org/10.1016/j.marpolbul.2007.02.009
Carey, R. O., K. W. Migliaccio, and M. T. Brown. 2011. “Nutrient Discharges to Biscayne Bay, Florida: Trends, Loads, and a Pollutant Index.” Science of the Total Environment 409 (3): 530–539. https://doi.org/10.1016/j.scitotenv.2010.10.029
Carlson Jr, P. R., L. A. Yarbro, and T. R. Barber. 1994. “Relationship of Sediment Sulfide to Mortality of Thalassia testudinum in Florida Bay.” Bulletin of Marine Science 54 (3): 733–746. https://www.researchgate.net/publication/233522335_Relationship_of_Sediment_Sulfide_to_Mortality_of_Thalassia_Testudinum_in_Florida_Bay
Chambers, L. G., H. E. Steinmuller, and J. L. Breithaupt. 2019. "Toward a Mechanistic Understanding of 'Peat Collapse' and Its Potential Contribution to Coastal Wetland Loss." Ecology 100 (7): e02720. https://doi.org/10.1002/ecy.2720
Doering, P. H., R. H. Chamberlain, and J. M. McMunigal. 2001. "Effects of Simulated Saltwater Intrusions on the Growth and Survival of Wild Celery, Vallisneria americana, from the Caloosahatchee Estuary (South Florida)." Estuaries 24: 894–903. https://doi.org/10.2307/1353180
Durako, M. J., M. O. Hall, and M. Merello. 2001. “Patterns of Change in the Seagrass Dominated Florida Bay Hydroscape.” In The Everglades, Florida Bay, and Coral Reefs of the Florida Keys: An Ecosystem Sourcebook, 479-496. CRC Press. http://dx.doi.org/10.1201/9781420039412-23
Feyrer, F., J. E. Cloern, L. R. Brown, M. A. Fish, K. A. Hieb, and R. D. Baxter. 2015. “Estuarine fish communities respond to climate variability over both river and ocean basins.” Global Change Biology 21 (10): 3608–3619. https://doi.org/10.1111/gcb.12969
Fisch, N. C., and W. E. Pine. 2016. “A Complex Relationship between Freshwater Discharge and Oyster Fishery Catch per Unit Effort in Apalachicola Bay, Florida: An Evaluation from 1960 to 2013.” Journal of Shellfish Research 35 (4): 809–825. https://doi.org/10.2983/035.035.0409
Ford, A. K., S. Bejarano, M. M. Nugues, P. M. Visser, S. Albert, and S. C. A. Ferse. 2018. "Reefs Under Siege – The Rise, Putative Drivers, and Consequences of Benthic Cyanobacterial Mats." Frontiers in Marine 5. https://doi.org/10.3389/fmars.2018.00018
Ford, A. K., P. M. Visser, M. J. van Herk, E. Jongepier, and V. Bonito. 2021. "First Insights into the Impacts of Benthic Cyanobacterial Mats on Fish Herbivory Functions on a Nearshore Coral Reef." Scientific Reports 11: 7147. https://doi.org/10.1038/s41598-021-84016-z
Green, D. P., J. C. Trexler, J. J. Lorenz, C. C. McIvor, and T. Philippi. 2006. “Spatial Patterns of Fish Communities along Two Estuarine Gradients in Southern Florida.” Hydrobiologia 569: 387–399. https://doi.org/10.1007/s10750-006-0144-x
Hall, M. O., B. T. Furman, M. Merello, and M. J. Durako. 2016. “Recurrence of Thalassia testudinum Seagrass Die-off in Florida Bay, USA: Initial Observations.” Marine Ecology Progress Series 560: 243–249. https://doi.org/10.3354/meps11923
Harlem, P. W. 1979. Aerial Photographic Interpretation of the Historical Changes in Northern Biscayne Bay, Florida: 1925 to 1976. Sea Grant Technical Bulletin 40. Miami, FL: University of Miami. Accessed December 2021. http://palmm.digital.flvc.org/islandora/object/fiu%3A29813
Havens, K. E. 2015. "Climate Change and the Occurrence of Harmful Microorganisms in Florida’s Ocean and Coastal Waters: SGEF216/SG136, 6/2015." EDIS 2015 (5): 6. https://doi.org/10.32473/edis-sg136-2015
IPCC. 2019. “Summary for Policymakers.” In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, edited by H.- O. Pörtner, D. C. Roberts, V. Masson-Delmotte, P. Zhai, M. Tignor, E. Poloczanska, K. Mintenbeck, M. Nicolai, A. Okem, J. Petzold, B. Rama, and N. Weyer, 3–35. Cambridge, UK: Cambridge University Press. https://doi.org/10.1017/9781009157964.001
Jenkins, G. P., S. D. Conron, and A. K. Morison. 2010. “Highly variable recruitment in an estuarine fish is determined by salinity stratification and freshwater flow: Implications of a changing climate.” Marine Ecology Progress Series 417: 249–261. https://doi.org/10.3354/meps08806
Johnson, F. A., E. V. Camp, R. Gandy, and W. E. Pine. 2023. “Demography of Oyster Pre- and Post-collapse in Apalachicola Bay, Florida, using Stage-based Counts.” Marine and Coastal Fisheries 15 (3): e10244. https://doi.org/10.1002/mcf2.10244
Johnson, F. A., W. E. Pine III, and E. V. Camp. 2022. “A Cautionary Tale: Management Implications of Critical Transitions in Oyster Fisheries.” Canadian Journal of Fisheries and Aquatic Sciences 79 (8): 1269–1281. https://doi.org/10.1139/cjfas-2021-0133
Kimbro, D. L., J. W. White, H. Tillotson, N. Cox, M. Christopher, O. Stokes-Cawley, S. Yuan, T. J. Pusack, and C. D. Stallings. 2017. “Local and regional stressors interact to drive a salinization-induced outbreak of predators on oyster reefs.” Ecosphere 8 (11): e01992-15. https://doi.org/10.1002/ecs2.1992
Lefcheck, J. S., B. B. Hughes, A. J. Johnson, B. W. Pfirrman, D. B. Rasher, A. R. Smyth, B. L. Williams, M. W. Beck, and R. J. Orth. 2019. “Are coastal habitats important nurseries? A meta‐analysis.” Conservation Letters 12 (4): e12645. https://doi.org/10.1111/conl.12645
Lefler, F. W., D. E. Berthold, and H. D. Laughinghouse IV. 2021. "The Occurrence of Affixifilum gen. nov. and Neolyngbya (Oscillatoriaceae) in South Florida (USA), with the Description of A. floridanum Sp. Nov. and N. biscaynensis Sp. nov." Journal of Phycology 57 (1): 92–110. https://doi.org/10.1111/jpy.13065
Lirman, D., and W. P. Cropper, Jr. 2003. "The Influence of Salinity on Seagrass Growth, Survivorship, and Distribution within Biscayne Bay, Florida: Experimental and Modeling Studies." Estuaries 26: 131–141. https://doi.org/10.1007/BF02691700
Love, G., S. Baker, and E. V. Camp. 2021. “Oyster-Predator Dynamics and Climate Change: FA228, 09/2020.” EDIS 2021 (1). https://doi.org/10.32473/edis-fa228-2020
Love, G., A. Braswell, A. B. Collins, and E. V. Camp. 2022a. “How Oyster Reefs Can Affect Finfish Recruitment: FA250, 12/2022.” EDIS 2022 (6). https://doi.org/10.32473/edis-fa250-2022
Love, G., A. Braswell, A. B. Collins, and E. V. Camp. 2022b. “Ecological Influences on Coastal Finfish Recruitment: FA239, 5/2022.” EDIS 2022 (3). https://doi.org/10.32473/edis-fa239-2022
Meeder J. F, P. W. Harlem, and A. Renshaw. 2001. “Historic Creek Watershed Study Final Results: Year 1.”
Millette, N., C. Kelble, A. Linhoss, S. Ashby, L. Visser. 2019. “Using Spatial Variability in the Rate of Change of Chlorophyll a to Improve Water Quality Management in a Subtropical Oligotrophic Estuary.” Estuaries and Coasts 42: 1792–1803. https://doi.org/10.1007/s12237-019-00610-5
Murray, S. J., P. N. Foster, and I. C. Prentice. 2012. "Future Global Water Resources with Respect to Climate Change and Water Withdrawals as Estimated by a Dynamic Global Vegetation Model." Journal of Hydrology 448–449: 14–29. https://doi.org/10.1016/j.jhydrol.2012.02.044
Nordlie, F. G. 2003. “Fish Communities of Estuarine Salt Marshes of Eastern North America, and Comparisons with Temperate Estuaries of Other Continents.” Reviews in Fish Biology and Fisheries 13: 281–325. https://doi.org/10.1023/B:RFBF.0000033050.51699.84
Parker, M., W. Arnold, S. Geiger, P. Gorman, and E. Leone. 2013. "Impacts of Freshwater Management Activities on Eastern Oyster (Crassostrea virginica) Density and Recruitment: Recovery and Long-term Stability in Seven Florida Estuaries." Journal of Shellfish Research 32 (3): 695–708. https://doi.org/10.2983/035.032.0311
Peterson, B. J., C. M. Chester, F. J. Jochem, and J. W. Fourqurean. 2006. “Potential Role of Sponge Communities in Controlling Phytoplankton Blooms in Florida Bay.” Marine Ecology Progress Series 328: 93–103. https://doi.org/10.3354/meps328093
Petes, L. E., A. J. Brown, and C. R. Knight. 2012. "Impacts of Upstream Drought and Water Withdrawals on the Health and Survival of Downstream Estuarine Oyster Populations." Ecology and Evolution 2 (7): 1712–1724. https://doi.org/10.1002/ece3.291
Phlips, E. J., S. Badylak, J. Hart, D. Haunert, J. Lockwood, K. O-Donnell, D. Sun, P. Viveros, and M. Yilmaz. 2012. "Climatic Influences on Autochthonous and Allochthonous Phytoplankton Blooms in a Subtropical Estuary, St. Lucie Estuary, Florida, USA." Estuaries and Coasts 35: 335–352. https://doi.org/10.1007/s12237-011-9442-2
Rudnick, David T., Z. Chen, D. L. Childers, and T. D. Fontaine. 1999. “Phosphorus and Nitrogen Inputs to Florida Bay: The Importance of the Everglades Watershed.” Estuaries 22: 398–416. https://doi.org/10.2307/1353207
Serafy, J., K. Lindeman, T. Hopkins, and J. Ault. 1997. "Effects of Freshwater Canal Discharge on Fish Assemblages in a Subtropical Bay: Field and Laboratory Observations." Marine Ecology Progress Series 160: 161–172. https://doi.org/10.3354/meps160161
Sheffield, J., and E. F. Wood. 2008. "Projected Changes in Drought Occurrence under Future Global Warming from Multi-Model, Multi-Scenario, IPCC AR4 Simulations." Climate Dynamics 31: 79–105. https://doi.org/10.1007/s00382-007-0340-z
Shumway, S. 1996. “Natural Environmental Factors.” In The Eastern Oyster Crassostrea virginica, edited by V. S. Kennedy, R. I. E. Newell, and A. F. Eble, 467–513. College Park, MD: Maryland Sea Grant, University of Maryland. https://repository.library.noaa.gov/view/noaa/45763/noaa_45763_DS1.pdf
Sime, P. 2005. “St. Lucie Estuary and Indian River Lagoon Conceptual Ecological Model.” Wetlands 25: 898–907. https://doi.org/10.1672/0277-5212(2005)025[0898:SLEAIR]2.0.CO;2
Smyth, A. R., L. K. Reynolds, S. C. Barry, N. C. Stephens, J. T. Patterson, and E. V. Camp. 2022. “Ecosystem Services Provided by Living Shorelines: SL494, 5/2022.” EDIS (3). https://doi.org/10.32473/edis-ss707-2022
Turner, R. E. 2006. “Will lowering estuarine salinity increase Gulf of Mexico oyster landings?” Estuaries and Coasts 29: 345–352. https://doi.org/10.1007/BF02784984
Wallace, C., A. Braswell, M. Clarke, A. Ropicki, T. Wade, F. Asche, A. Smyth, A. Ubeda, and E. V. Camp. 2023. “How Ecosystem Services Are Measured and Why It Matters for Florida: FA252, 2/2023.” EDIS 2023 (1). https://doi.org/10.32473/edis-fa252-2023
Wallace, C., E. V. Camp, and A. Smyth. 2022. “Oyster Habitat Restoration and Shoreline Protection: FOR376/FR446, 3/2022.” EDIS 2022 (2). https://doi.org/10.32473/edis-fr446-2022
Wilson, B. J., S. Servais, S. P. Charles, S. E. Davis, E. E. Gaiser, J. S. Kominoski, J. H. Richards, and T. G. Troxler. 2018. “Declines in plant productivity drive carbon loss from brackish coastal wetland mesocosms exposed to saltwater intrusion.” Estuaries and Coasts 41: 2147–2158. https://doi.org/10.1007/s12237-018-0438-z
Wingard, L. G. 2004. “Changing Salinity Patterns in Biscayne Bay, Florida.” Fact Sheet 2004-3108. U.S. Geological Survey. https://pubs.usgs.gov/fs/2004/3108/report.pdf
Wood, S. A., L. T. Kelly, K. Bouma-Gregson, J.-F. Humbert, H. D. Laughinghouse IV, J. Lazorchak, T. G. McAllister, et al. 2020. "Toxic Benthic Freshwater Cyanobacterial Proliferations: Challenges and Solutions for Enhancing Knowledge and Improving Monitoring and Mitigation." Freshwater Biology 65 (10): 1824–1842. https://doi.org/10.1111/fwb.13532
Wuebbles, D., G. Meehl, K. Hayhoe, T. R. Karl, K. Kunkel, B. Santer, M. Wehner, et al. 2014. "CMIP5 Climate Model Analysis: Climate Extremes in the United States." Bulletin of the American Meteorological Society 95 (4): 571–583. https://doi.org/10.1175/BAMS-D-12-00172.1
Zieman, J. C., J. W. Fourqurean, and T. A. Frankovich. 1999. "Seagrass Die-Off in Florida Bay: Long-Term Trends in Abundance and Growth of Turtle Grass: Thalassia testudinum." Estuaries 22: 460–470. https://doi.org/10.2307/1353211.