Abstract
Recruitment is a very important life stage for fish, with direct impacts on the size of whole fish populations. During this phase, the mortality of juvenile fish is affected by their population density because of competition for the available resources. The degree to which fish population density affects mortality in recruitment is itself affected by many factors, but one of the most important is the structural habitat available to juvenile fish. Structurally complex habitats like oyster reefs are thought to have a particularly strong influence on the recruitment process of certain species. Here we describe the ways that habitat can alter recruitment success. We focus on how oyster reefs affect recruitment of fish in Florida and the particular issues related to the management of this important habitat.
Introduction
Recruitment is a critical life history stage for fish and aquatic invertebrates that directly influences the overall population status. During the recruitment phase, the mortality the young fish experience is often density-dependent (Lorenzen and Camp 2019). Density-dependent mortality refers to how mortality rates increase with increasing density of fish and decrease with decreasing fish density. Juvenile fish competing for resources results in density-dependent mortality. Fish that survive this phase are called “recruits.” Recruits join sub-adult and adult populations and generally their mortality is independent of density. This dynamic means that the number of fish that recruit each year directly affects the size of the adult population. So, the surviving recruit abundance has a large impact on the number of fish that mature to spawn and be fished, essentially affecting the future of the population. More information on recruitment can be found in FA222 and FA234, and an overview of the factors affecting recruitment, including habitat, can be found in Ecological Influences on Coastal Finfish Recruitment.
One of the most important ecological findings from the last 30 years is that the density-dependent mortality that fish go through during recruitment is affected by the habitat available to the fish (Walters and Juanes 1993). Fish habitat includes physical, structural habitat (like aquatic rocks or vegetation) that affects ecological processes like foraging and finding refuge from predators. By affecting competitive processes, like foraging and refuge-seeking, habitats can influence the intensity of density-dependent mortality. Through this process, habitat changes can alter recruitment levels and fish populations overall. In this publication, we describe ways in which habitat can alter recruitment processes, specifically with regard to oyster reefs and recent/ongoing losses of oyster habitat in Florida and beyond. Oysters have declined by about 90% globally, and while Florida oyster reefs may be in fair health, their condition has declined, exemplified by the collapse of the oyster population in Apalachicola Bay in 2012 (Beck et al. 2011; zu Ermgassen et al. 2012; Pine et al. 2015). This information should help the interested public understand the particular attention being paid to oyster preservation, conservation, and oyster reef restoration. It should also be helpful to management agency personnel and outreach professionals for use in educational efforts about habitat importance to fish populations—in particular, oyster reef habitat.

Credit: Florida Sea Grant
Habitat Influences on Recruitment
We discuss the environmental influences on the recruitment process in the publication, Ecological Influences on Coastal Finfish Recruitment; in this publication, we will expand on the topic and describe how habitat specifically alters these processes. One of the principles of ecology is that most animals, including fish, have preferred or required habitats. Many habitats that coastal finfish use are structural habitats, which are made up of physical, 3-dimensional materials above the substrate (e.g., rock, seagrass, mangroves, marsh grass, and oyster reefs). One of the key things these “complex” habitats have in common is that they change the space from expansive open waters to more varied habitat that offers “interstitial spaces,” the small nooks between the structures (Dibble et al. 1996). Little or no habitat favored by fish leaves large open spaces. Conversely, structural habitat creates many small spaces in the openings and nooks between the structures. One way to think about this is to imagine a 10-gallon rectangular fish aquarium tank that pet fish are kept in. If no plants, rocks, or anything else are added to the tank, the fish experience a singular, wide-open space. As soon as structures like wood, plants, ceramic caves, or rocks are added, that space is broken into many small spaces of different shapes. In the wild, structure is actually important to many fish, but the small interstitial spaces are especially important for the variety of young, small fish that use the structural habitats and that tend to have many predators, such as crabs, birds, and larger fish (Figure 1). These small young fish hide or “take refuge” in the small habitat spaces where predators cannot find or reach them (Savino and Stein 1982). The young fish forage effectively within the safe interstitial spaces until they can grow large enough to escape size-based predation (Mittelbach 1982; Sogard 1997). These complex habitats and their small interstitial spaces improve survival for young fish, so they are often called nursery habitats (Beck et al. 2001).
So, if structural habitat creates small interstitial spaces that are used by young fish for foraging and hiding, what happens if habitat changes? In short, changes in the amount of space influences competition among the small fish using them. In other words, the intensity of density-dependent mortality depends on structural habitat availability (Walters and Juanes 1993). This effect can be illustrated with the following example. Imagine there are 100 good nooks for hiding in a certain oyster reef, and there are 1,000 juvenile gulf toadfish looking for a good place to hide. If each space fits only 1 fish, then 100 fish can find good refuge space. The remaining 900 that do not have a good hiding space will likely be at greater risk of being eaten by predators and experience more mortality. In the next year, if there are only 200 small gulf toadfish vying for the same 100 hiding places, a much greater proportion of the fish will find a place to hide. This example is density-dependent mortality, where the mortality rate changes depending on how many fish are competing for the same resources. In the example above, if we assumed all fish that did not have a good hiding space did in fact get eaten, the juvenile mortality rate would be 90% in the first year (900/1000 fish died), but only 50% (100/200 fish died) in the second year. This example shows how fish population density affects mortality. Now let us imagine the habitat is degraded. If half of the oyster bar and hiding places are lost, the competition for the remaining 50 spots will increase, and mortality will increase with it. We could expect about 950/1000 or 95% mortality in the first year, and 150/200 or 75% in the second year. This example is simply illustrated in Figure 2.
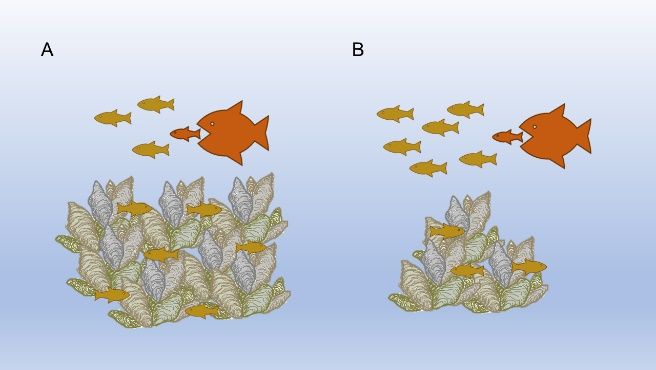
Credit: Gabrielle Love
Structural habitats like oyster reefs are important to small fish not only because they provide hiding spaces. Small fish also need to eat so they can get bigger and avoid predators. The problem is almost all animals, including small fish, are more vulnerable when they are foraging (instead of hiding). What the small fish really need is good hiding spaces that are very close to good foraging spaces. These places let them reduce their exposure to predators while still getting food to grow. This intuitive concept is described by the “foraging arena theory”. This theory is an additional mechanistic understanding of how habitat is affected by density-dependent mortality (Walters and Juanes 1993; Ahrens et al. 2012). Foraging arena theory recognizes that most waters are made up of different foraging “arenas,” areas where smaller fish look for forage and in doing so are exposed to predation from larger fish. The theory describes how habitats, especially structural habitats, can change the “arena.” More structural habitat may make it easier for small fish to forage without being preyed upon, and less may make it riskier to forage. The theory goes on to specify how rates of movement between refuge and feeding areas can be used to understand effects on mortality and, ultimately, populations. The main point is that we recognize that structural habitat, like the kind provided in many coastal systems, is really critical for determining how impactful density-dependent mortality is.
Habitat can also influence the density-independent causes of recruitment mortality, like water flow. Larval fish rely on water flow patterns to transport them to suitable habitats, and the shape of structural habitat can itself alter the small-scale water flow. This effect on flow can impact the food availability for and movement of very young fish, affecting settlement and survival (Breitburg 1991). Water depth and tides change how fish interact with a habitat space. This change is especially common with oyster reefs in estuaries, where tidal variation causes some reefs to be periodically exposed to the air, while others are constantly underwater. The height of an underwater habitat changes the relative water depth experienced by species using the habitat. The differences in depth change the water flow and movement of floating sediment and/or food. These can change the available food supply and can make a habitat more or less desirable for fish (Lenihan et al. 2001; Stunz et al. 2002a; Nevins et al. 2014).
Species of Interest
Oyster reefs are used as habitat by a wide range of species. For some, oyster reefs serve as nursery habitat only and are not necessary for adults of that species. Other species are lifelong residents of oyster reefs. Entire food chains are sustained by oyster reefs, as oysters are food for many crabs and fish (Harding 1999; Coen and Grizzle 2007). Other small invertebrates inhabit the reefs and attract even more predators than oysters do (Coen and Grizzle 2007; Shervette and Gelwick 2008). Smaller predators, like mud crabs, blue crabs, blennies, and gobies, often become prey for larger fish. These include gray snapper, black sea bass, red drum, sheepshead, gag grouper, and striped bass (Tolley and Volety 2005; Coen and Grizzle 2007; Yeager and Layman 2011; Pierson and Eggleston 2014; Harding et al. 2015). These species could be affected by changes to oyster reef habitat altering recruitment somewhere along the food chain, even if a particular fish species does not experience recruitment on oyster reefs directly.
Some fish species use oyster reefs specifically for recruitment (Table 1). Gray snapper recruit directly onto oyster reef habitats and rely on them for most of their food as juveniles (Peterson et al. 2003; Yeager and Layman 2011). Gag grouper recruit to oyster reefs as juveniles before migrating offshore as adults (Peterson et al. 2003). Species like black sea bass, striped bass, and spot forage heavily on oyster reefs as juveniles (Coen and Grizzle 2007). These species are particularly vulnerable to oyster reef degradation or loss (Beck et al. 2001).
Table 1. Examples of species that recruit to oyster reefs in Florida.
Oyster Reefs and Alternatives
Some species can use a few different habitat options as nursery habitat. In estuaries, seagrasses and salt marshes are often the most abundant alternative to oyster reefs. Juveniles will often select a particular habitat to optimize feeding and to minimize predation risk and competition (Stunz et al. 2002). Red drum, for example, tend to prefer to recruit in seagrasses, but mortality is lower on oyster reefs (Stunz and Minello 2001). If seagrass habitat becomes degraded, however, oyster reefs become extremely important (Coen and Grizzle 2007). As habitats in general become degraded, a high quantity of available alternative habitat spaces is necessary to maintain fish recruit numbers (Rosenfeld 2002). The loss of oyster reef habitat decreases the usable refuge and foraging spaces, and it decreases the number of fish that can successfully survive recruitment. The degraded quality of reefs can also reduce their viability as nursery habitat. Whether it’s by shorter reef height changing water flow or by increased competition from the reduction in usable space, reef quality is an important factor influencing the ability of fish to survive and successfully recruit (Rosenfeld and Hatfield 2006).
Table 2. Species expected to experience enhanced recruitment and population abundances as a result of oyster reef restoration. From zu Ermgassen et al. (2017).
Conclusions
Because recruitment is such an important process for the health of fish populations, nursery habitats must be carefully protected. The recruitment behaviors of a lot of fish species in Florida are still not fully understood, and oyster reefs may be important nursery habitat for more species than we currently know. Since habitat degradation limits the number of young fish, increasing habitat availability is expected to enhance recruitment, and therefore the overall populations, of many species (Powers et al. 2003, zu Ermgassen et al. 2017). Several of these are listed in Table 2. Efforts to conserve and restore oyster reefs could have far-reaching benefits for entire food chains and are important tools for fisheries management in Florida.
References
Ahrens, R. N. M., C. J. Walters, and V. Christensen. 2012. “Foraging Arena Theory.” Fish and Fisheries 13 (1): 41–59. https://doi.org/10.1111/j.1467-2979.2011.00432.x
Beck, M. W., K. L. Heck, K. W. Able, D. L. Childers, D. B. Eggleston, B. M. Gillanders, B. Halpern, B., et al. 2001. “The Identification, Conservation, and Management of Estuarine and Marine Nurseries for Fish and Invertebrates.” BioScience 51 (8): 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Beck, M. W., R. Brumbaugh, L. Airoldi, A. Carranza, L. Coen, C. Crawford, O. Defeo, et al. 2011. “Oyster Reefs at Risk and Recommendations for Conservation, Restoration, and Management.” BioScience 61 (2): 107–116. https://doi.org/10.1525/bio.2011.61.2.5
Breitburg, D. L. 1991. “Settlement Patterns and Presettlement Behavior of the Naked Goby, Gobiosoma bosci, a Temperate Oyster Reef Fish.” Marine Biology 109 (2): 213–221. https://doi.org/10.1007/BF01319389
Breitburg, D., M. Palmer, and T. Loher. 1995. “Larval Distributions and the Spatial Patterns of Settlement of an Oyster Reef Fish: Responses to Flow and Structure.” Marine Ecology Progress Series 125:45–60. https://doi.org/10.3354/meps125045
Coen, L. D., and R. E. Grizzle. 2007. The Importance of Habitat Created by Molluscan Shellfish to Managed Species along the Atlantic Coast of the United States (Habitat Management Series #8) [ASMFC Habitat Management Series]. Atlantic States Marine Fisheries Committee.
Dibble, E. D., K. J. Killgore, and G. O. Dick. 1996. “Measurement of Plant Architecture in Seven Aquatic Plants.” Journal of Freshwater Ecology 11 (3): 311–318. https://doi.org/10.1080/02705060.1996.9664453
Harding, J. M. 1999. “Selective Feeding Behavior of Larval Naked Gobies Gobiosoma bosc and Blennies Chasmodes bosquianus and Hypsoblennius hentzi: Preferences for Bivalve Veligers. Marine Ecology Progress Series 179:145–153. https://doi.org/10.3354/meps179145
Harding, J. M., D. M. Allen, S. Dingley, R. M. Tremont, S. M. Krug, and C. T. Armstrong. 2015. “Ontogenetic Changes in Predator–Prey Interactions between Two Species of Larval Fishes and Oyster Veligers.” Journal of Experimental Marine Biology and Ecology 471:164–174. https://doi.org/10.1016/j.jembe.2015.06.004
Holling, C. S. 1959. “The Components of Predation as Revealed by a Study of Small-Mammal Predation of the European Pine Sawfly.” The Canadian Entomologist 28.
Lenihan, H. S., C. H. Peterson, J. E. Byers, J. H. Grabowski, G. W. Thayer, and D. R. Colby. 2001. “Cascading of Habitat Degradation: Oyster Reefs Invaded by Refugee Fishes Escaping Stress.” Ecological Applications 11 (3): 764–782. https://doi.org/10.1890/1051-0761(2001)011[0764:COHDOR]2.0.CO;2
Lorenzen, K., and E. V. Camp. 2019. “Density-Dependence in the Life History of Fishes: When Is a Fish Recruited? Fisheries Research 217:5–10. https://doi.org/10.1016/j.fishres.2018.09.024
Mittelbach, G. G. 1981. “Foraging Efficiency and Body Size: A Study of Optimal Diet and Habitat Use by Bluegills.” Ecology 62 (5): 1370–1386.
Nevins, J. A., J. B. Pollack, and G. W. Stunz. 2014. “Characterizing Nekton Use of the Largest Unfished Oyster Reef in the United States Compared with Adjacent Estuarine Habitats.” Journal of Shellfish Research 33 (1): 227–238. https://doi.org/10.2983/035.033.0122
Peterson, C. H., J. H. Grabowski, and S. P. Powers. 2003. “Estimated Enhancement of Fish Production Resulting from Restoring Oyster Reef Habitat: Quantitative Valuation.” Marine Ecology Progress Series 264:249–264. https://doi.org/10.3354/meps264249
Pierson, K. J., and D. B. Eggleston. 2014. “Response of Estuarine Fish to Large-Scale Oyster Reef Restoration.” Transactions of the American Fisheries Society 143 (1): 273–288. https://doi.org/10.1080/00028487.2013.847863
Pine, W., C. Walters, E. Camp, R. Bouchillon, R. Ahrens, L. Sturmer, and M. Berrigan. 2015. “The Curious Case of Eastern Oyster Crassostrea virginica Stock Status in Apalachicola Bay, Florida.” Ecology and Society 20(3). https://doi.org/10.5751/ES-07827-200346
Powers, S. P., J. H. Grabowski, C. H. Peterson, and W. J. Lindberg. 2003. “Estimating Enhancement of Fish Production by Offshore Artificial Reefs: Uncertainty Exhibited by Divergent Scenarios.” Marine Ecology Progress Series 264:265–277. https://doi.org/10.3354/meps264265
Rosenfeld, J. S. 2002. “Functional Redundancy in Ecology and Conservation.” Oikos 98 (1): 156–162. https://doi.org/10.1034/j.1600-0706.2002.980116.x
Rosenfeld, J. S., and T. Hatfield. 2006. “Information Needs for Assessing Critical Habitat of Freshwater Fish.” Canadian Journal of Fisheries and Aquatic Sciences 63 (3): 683–698. https://doi.org/10.1139/f05-242
Savino, J. F., and R. A. Stein. 1982. “Predator-Prey Interaction between Largemouth Bass and Bluegills as Influenced by Simulated, Submersed Vegetation.” Transactions of the American Fisheries Society 111 (3): 255–266.
Shervette, V. R., and F. Gelwick. 2008. “Relative Nursery Function of Oyster, Vegetated Marsh Edge, and Nonvegetated Bottom Habitats for Juvenile White Shrimp Litopenaeus setiferus.” Wetlands Ecology and Management 16:405–419. https://doi.org/10.1007/s11273-007-9077-z
Sogard, S. M. 1997. “Size-Selective Mortality in the Juvenile Stage of Teleost Fishes: A Review.” Bulletin of Marine Science 60 (3): 1129–1157.
Stunz, G. W., and T. J. Minello. 2001. “Habitat-Related Predation on Juvenile Wild-Caught and Hatchery-Reared Red Drum Sciaenops ocellatus (Linnaeus).” Journal of Experimental Marine Biology and Ecology 260:13–25.
Stunz, G. W., T. J. Minello, and P. S. Levin. 2002. “A Comparison of Early Juvenile Red Drum Densities among Various Habitat Types in Galveston Bay, Texas.” Estuaries 25 (1): 76–85. https://doi.org/10.1007/BF02696051
Tolley, S. G., and A. K. Volety. 2005. “The Role of Oysters in Habitat Use of Oyster Reefs by Resident Fishes and Decapod Crustaceans.” Journal of Shellfish Research 24 (4): 1007–1012. https://doi.org/10.2983/0730-8000(2005)24[1007:TROOIH]2.0.CO;2
Tolley, S. G., A. K. Volety, and M. Savarese. 2005. “Influence of Salinity on the Habitat Use of Oyster Reefs in Three Southwest Florida Estuaries.” Journal of Shellfish Research 24 (1): 127–137. https://doi.org/10.2983/0730-8000(2005)24[127:IOSOTH]2.0.CO;2
Walters, C. J., and F. Juanes. 1993. “Recruitment Limitation as a Consequence of Natural Selection for Use of Restricted Feeding Habitats and Predation Risk Taking by Juvenile Fishes.” Canadian Journal of Fisheries and Aquatic Sciences 50 (10): 2058–2070. https://doi.org/10.1139/f93-229
Yeager, L. A., and C. A. Layman. 2011. “Energy Flow to Two Abundant Consumers in a Subtropical Oyster Reef Food Web.” Aquatic Ecology 45:267–277. https://doi.org/10.1007/s10452-011-9352-1
zu Ermgassen, P. S. E., M. D. Spalding, B. Blake, L. D. Coen, B. Dumbauld, S. Geiger, J. H. Grabowski, et al. 2012. “Historical Ecology with Real Numbers: Past and Present Extent and Biomass of an Imperilled Estuarine Habitat.” Proceedings of the Royal Society B: Biological Sciences 279 (1742): 3393–3400. https://doi.org/10.1098/rspb.2012.0313
zu Ermgassen, P., B. Hancock, B. DeAngelis, J. Greene, E. Schuster, M. Spalding, and R. Brumbaugh. 2017. Setting Objectives for Oyster Habitat Restoration Using Ecosystem Services: A Manager’s Guide. The Nature Conservancy. http://www.oyster-restoration.org/setting-objectives-for-oyster-habitat-restoration-using-ecosystem-services-a-managers-guide/