Introduction
Fertigation is the process of applying fertilizers through an irrigation system, such as a drip, sprinkler, or pivot system, by injecting selected fertilizers into the water. Because of its effectiveness and efficiency, fertigation is widely used in vegetable and fruit production. However, clogging of lines and emitters may become a problem (Figures 1 and 2) if not managed appropriately. This publication provides practical suggestions for fruit and vegetable growers to better manage fertigation and reduce clogging problems.

Credit: Guodong Liu, UF/IFAS

Credit: Guodong Liu, UF/IFAS
Why does clogging occur?
Since fertigation is a combination of irrigation and fertilization, clogging problems can be traced back to either the water supply or injected fertilizer.
Water
The primary reason for clogging associated with water is poor water quality. Water quality includes the physical, chemical, and biological qualities of irrigation water. Please refer to Causes and Prevention of Emitter Plugging in Microirrigation Systems (https://edis.ifas.ufl.edu/ae032) for additional information.
Physical: The irrigation water can have too many suspended solids, such as debris and tiny clay particles, which can plug irrigation lines and emitters. When suspended solids exceed 50 parts per million (ppm) in the irrigation water, clogging problems are likely. The clogging problem will be severe if suspended solids are in excess of 100 ppm (Table 1). Please refer to Irrigation and Household Water Test and Interpretation (https://edis.ifas.ufl.edu/ss440) for more information.
Chemical: Chemical problems include high pH and high concentrations of cations and/or anions in the irrigation water. In the presence of high pH (pH greater than 5.3 [Obreza, Hanlon, and Zekri 2011]) and oxygen within the water, the ferrous iron species is vulnerable to oxidation and creates a ferric iron precipitate. This oxidization not only reduces the bioavailability of iron applied through fertigation but also creates clogging problems. Generally speaking, when the water's pH is greater than 5.3, the ferrous iron species can be oxidized. Studies indicate that approximately 50% of the ferrous iron species can be oxidized to ferric iron at pH 6.3 in 20 minutes (Morgan and Lahov 2007). The higher the pH, the more serious the clogging problem if the irrigation water has a total iron concentration greater than 0.2 ppm (Table 1). Irrigation with "hard" water, which is high in minerals such as calcium and magnesium, can also cause clogging problems because the calcium and magnesium ions are susceptible to precipitation with carbonates and phosphates. Please refer to Water Quality Note: Alkalinity and Hardness (https://edis.ifas.ufl.edu/ss540) for more information.
Biological: Bacterial growth can cause clogging problems when the bacterial population is greater than 2,600 colony-forming units (CFU) per gallon. When the number exceeds 13,200 CFU per gallon, bacteria in the water may cause severe clogging problems (Table 1). Water sources rich in nutrients have greater potential to support the growth of bacteria and clog irrigation systems. The bacteria oxidize iron and manganese and initiate the slime-like growth of biofilms, which can clog the system. Iron and manganese oxides can also form without bacteria, but when bacteria are present, bacterial formation of the oxides dominates (Burt, O'Connor, and Ruehr 1995). Finally, the presence of algae in surface water supplies can also clog fertigation systems, as can root intrusion into buried drip emitters.
Fertilizer
The effect of different fertilizers on clogging is statistically significant (Bozkurt and Ozekici 2006). Some fertilizers can also cause clogging problems if they are incompatible with fertigation. Chemical reactions may occur after the fertilizers are injected into the irrigation system. Additional problems can occur with regard to the temperature of the mixing water, the coagulation and secondary chemical reactions in the mixing tank, or failure to keep suspensions for a number of reasons, as shown below.
Fertilizer incompatibility: For example, calcium nitrate and diammonium phosphate are fertilizers commonly used for commercial crop production, but these two fertilizers are NOT compatible. If these fertilizers are mixed together, calcium from calcium nitrate and phosphate from diammonium phosphate can form calcium phosphate, which is insoluble and precipitates out, clogging lines and emitters during fertigation.
Chemical reactions: There may be additional chemical reactions after the selected fertilizers are injected into irrigation water. These reactions include hydrolysis, dissociation, oxidization, and precipitation. The first two types of reactions can accelerate the second two types of reactions. These chemical reactions can all cause clogging.
Hydrolysis: When urea or a fertilizer containing urea, e.g., UAN-32 is injected into the irrigation water, the following hydrolytic reaction occurs. This reaction produces ammonia and carbon dioxide, as the following urease-accelerated enzymatic equation shows:
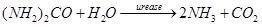
Dissociation: After the above hydrolysis of urea, the two products, ammonia and carbon dioxide, dissociate in water immediately, as shown below:
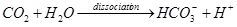
Carbon dioxide is a very weak acidic gas. After carbon dioxide is dissociated, the acidity of the irrigation water can be lowered a little bit to pH 6.4. However, compared with the acidity of carbon dioxide, the alkalinity of ammonia is approximately a hundredfold stronger. Accordingly, the dissociation of ammonia generates hydroxyl ions and raises the pH to 9.3. Particularly, ammonia in urea is stoichiometrically twice the concentration of that of carbon dioxide. Thus, as a net result of urea dissolved in water, the resulting pH can be greater than 9.0.
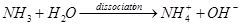
Urea has the potential to generate a considerable amount of hydroxyl ions, increasing water pH. This hydroxide formation can accelerate oxidization of micronutrients, such as iron and manganese. Iron and manganese oxides may form accordingly. Ferric and manganese oxides/hydroxides are insoluble in water. In addition, the bicarbonate ions from the hydrolysis may react to form calcium carbonate and magnesium carbonate because there is a dynamic equilibrium between bicarbonate and carbonate. The concentration of carbonate increases with bicarbonate concentration for the same reason. These carbonates can contribute to slime-like deposits inside irrigation lines and emitters.
Potassium thiosulfate (K2S2O3) and potassium polysulfide (KSx) are liquid fertilizers frequently used in fertigation. Potassium thiosulfate is 0-0-25-17S and potassium polysulfide 0-0-22-23S. These numbers represent percentages of nitrogen, phosphorus pentoxide, potassium oxide, and sulfur. For example, 0-0-25-17S means that fertilizer has 0% N and P but 25% potassium oxide and 17% sulfur. These solutions have high pH because of the dissociation of thiosulfate and polysulfide and may cause clogging problems, as described above, if proper steps are not taken. Therefore, the above two liquid fertilizers should not mix with iron and manganese fertilizers nor with calcium and magnesium fertilizers.
Oxidization: Only the form of ferrous iron, Fe(II), is bioavailable to fruit and vegetable plants. Because of the great reduction-oxidation potential difference between ferrous iron and oxygen, particularly at high pH, ferrous iron is very susceptible to being oxidized to ferric iron (Scott and Renaud 2007), which usually forms insoluble compounds with ferric hydroxide or ferric phosphate. The ferric iron species contributes to slime-like growth inside the fertigation system.
Precipitation: Ferric iron can precipitate with different anions, such as hydroxyl, phosphate, and sulfide, when it is formed. At neutral pH, ferric iron can be precipitated at less than 0.1 ppm. As described, urea may increase water pH to greater than 9 after hydrolysis. At this high pH, both calcium and magnesium are readily precipitated with carbonate or hydroxyl ions. All of these precipitations cause slime-like deposits and intensify clogging problems.
How can clogging be reduced?
Filter Irrigation Water
Good-quality irrigation water is crucial to reducing clogging problems in fertigation systems. If the irrigation water has visible debris and/or algae, irrigation water filters should be used to improve the quality of irrigation water and remove debris, clay particles, and algae before they enter the fertigation system.
Acidify Irrigation Water
When water pH is high, a suitable acid is needed to acidify the water source (Kidder and Hanlon 2012). Safety is always the top priority when irrigation water is acidified. When acidifying a water source, acid must always be added to water, but water must NEVER be put into acid. There are different acids, such as hydrochloric, sulfuric, citric, and phosphoric. Acidic fertilizers can also acidify irrigation water. For example, urea-sulfuric (N-pHURIC® products) nitrogen fertilizers can provide acidification and nutrients, including nitrogen, sulfur, etc., and lower water pH. Urea-sulfuric fertilizers are NOT compatible with many compounds. Growers should NEVER combine urea-sulfuric fertilizers with other fertilizers or compounds when acidifying irrigation water.
When citric acid or phosphoric acid is chosen, more acid is needed than either hydrochloric acid or sulfuric acid because both citric acid and phosphoric acid can build pH buffer systems in the irrigation water. Economically, urea-sulfuric fertilizers, hydrochloric acid, or sulfuric acid may be more effective (Kidder and Hanlon 2012). Special care should be taken when phosphoric acid is injected into the irrigation water because it may be precipitated out with calcium in the water. When calcium concentration is more than 50 ppm, phosphoric acid should NOT be injected (Burt, O'Connor, and Ruehr 1995). Calcium- or iron-fouled emitters can be cleaned by soaking emitters in a 0.5%–1.0% citric acid solution for 24–48 hours (Runyan et al. 2007).
Acidic solutions can damage irrigation system hardware. Corrosion-resistant fittings and components should be used if acids are to be injected. Acidifying irrigation water to pH 3.0 should NOT be longer than 30 minutes (Burt, O'Connor, and Ruehr 1995). Cleaning the fouled lines and emitters does NOT need to dissolve the buildup scales or slime inside the lines or emitters, but it does need to break them up and flush them out. Following irrigation water acidification, the irrigation system should be flushed for half an hour to ensure that the injected acid is completely cleaned out of the system (Obreza, Hanlon, and Zekri 2011).
Chlorinate Irrigation Water
To effectively prevent bacterial growth in irrigation water, chlorine may be injected continuously at low levels. Either liquid or gas chlorine can be used. Sodium hypochlorite (NaOCl) solution (household bleach) is also readily available. At a concentration of 5 ppm or lower, active chlorine, and not chloride, can effectively kill bacteria in irrigation water. After chlorine application, the free chlorine concentration should be measured at the hose ends toward the completion of an irrigation set. For irrigation water with high bacteria populations, a continuous application may be needed and 0.5–1.0 ppm free available chlorine should persist at the ends of lines. For irrigation water with relatively low bacterial populations, intermittent application of chlorine may be used. For occasional application, a concentration of approximately 5 ppm should be measured at the ends of lines. Regarding the bacterial density in the irrigation water, surface water has the potential to have greater bacterial population than deep well water.
Ensure that Injected Nutrients are Compatible
Fertilizer compatibility problems may occur when mixing two liquid fertilizers or liquid fertilizers with solid fertilizers. Before preparing fertilizer solutions from different sources, growers must consider the following points to ensure compatibility (Burt, O'Connor, and Ruehr 1995):
- The operator's safety when making the fertilizer solutions
- The likelihood of the fertilizers clogging the fertigation system
- The effects of the solutions on each other when mixed
- The reactions of the fertilizers injected into the irrigation system
If there is uncertainty about the safety of a fertilizer solution or the compatibility of its ingredients, growers should consult their county Extension agent or fertilizer specialist before proceeding. If both cations and anions can combine to form an insoluble compound, then the sources are NOT compatible. If the newly formed compound is slightly soluble, the grower needs to be very careful because fertilizers with low solubility properties can cause clogging problems (Table 2).
To test the compatibility of a fertilizer solution with different ingredients, the best way is to prepare a small quantity of the solution before mixing various fertilizer solutions and performing fertigation. This test is called a "jar test" (Boman and Obreza 2012; Obreza, Hanlon, and Zekri 2011). To perform a jar test, put some of the fertilizer solution into a jar of irrigation water and then carefully watch for any cloudiness for approximately 2 hours. If milkiness occurs, then the injection of the fertilizer solution will cause clogging problems. If two different fertilizer solutions are to be injected into the irrigation system, mix them in a jar. When performing a jar test, use the approximate dilution rate that would be used for the actual fertigation application. For example, if a fertilizer solution is to be injected at the rate of 15 gallons per hour into an irrigation system that delivers 600 gallons per minute to the crop, the dilution rate would be 1 to 2400. For more information, please refer to How to Calculate Fertigation Injection Rates for Commercial Blueberry Production (https://edis.ifas.ufl.edu/hs1197). To run the jar test for fertigation, 1 teaspoon (5 milliliters) of fertilizer solution put into 3 gallons (about 12000 milliliters) of water should be the same as the field injection rate.
If roots are causing buried emitters to clog, then the emitters need to be properly cleaned or replaced.
Practical Take-Home Message
- Clogging of irrigation lines and emitters is a common problem in fertigation. The problem can be reduced by improving the quality of irrigation water and injecting fertilizers judiciously.
- Use of irrigation water filters can significantly improve the quality of irrigation water and reduce clogging problems when water sources contain particulate matter such as debris or algae.
- Acidifying water sources can minimize oxidization of iron and manganese and reduce chemical precipitation between cation and anion nutrients.
- When acidification of a water source is required, acid must always be added to water, but water must NEVER be put into acid.
- Chlorination of the water source is necessary for irrigation water containing high bacterial populations to prevent the formation of bacterial slimes.
- Performing a "jar test" for fertilizer compatibility is strongly encouraged before mixing fertilizers for the first time in the mixing tank for fertigation.
References
Bozkurt, S. and B. Ozekici. 2006. "Effects of Fertigation Managements on Clogging of In-Line Emitters." Journal of Applied Science 6 (15): 3026–3034.
Bucks, D. A. and F. S. Nakayama. 1980. "Injection of Fertilizer and Other Chemicals for Drip Irrigation." In Proceedings of the Agri-Turf Irrigation Conference, Houston, Texas, 166–180. Silver Spring, MD: The Irrigation Association.
Burt, C., K. O'Connor, and T. Ruehr. 1995. Fertigation. San Luis Obispo: California Polytechnic State University.
Liu, G. D., E. H. Simonne, and Y. Li. 2011. Nickel Nutrition in Plants. HS1191. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/hs1191.
Morgan, B. and O. Lahov. 2007. "The Effect of pH on the Kinetics of Spontaneous Fe(II) Oxidation by O2 in Aqueous Solution – Basic Principles and a Simple Heuristic Description." Chemosphere 68 (11): 2080–2084.
Obreza, T., E. Hanlon, and M. Zekri. 2021. Dealing with Iron and Other Micro-Irrigation Plugging Problems. SL 265. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/ss487.
Runyan, C., T. Obreza, T. Tyson, B. Goodman, P. Tacker, R. Yager, J. Thomas, et. al. 2007. "Maintenance Guide for Microirrigation Systems in the Southern Region." FAWN Irrigation Maintenance Guide. http://fawn.ifas.ufl.edu/tools/irrigation/citrus/maintenance/FAWN%20Irrigation%20Maintenance%20Guide.pdf.
Scott, H. D. and F. G. Renaud. 2007. "Aeration and Drainage." In Irrigation of Agricultural Crops (2nd ed.), edited by R. J. Lascano and R. E. Sojka, 195–235. Madison, WI: ASA-CSA-SSSA.
The relationship between water characteristics and the hazard level for clogging during fertigation.