What is salinity?
Salinity is defined as the salt concentration of seawater, or the total amount of salts, in grams, dissolved in 1,000 grams (1 kilogram, about 1 liter) of fresh water. Therefore, it can be expressed as grams per liter or parts per thousand (or ppt). Seawater contains almost every known naturally occurring element, but about 86% of the ions in seawater are sodium chloride, or ordinary table salt. The other major dissolved ions include sulfate, magnesium, calcium, potassium, and bicarbonate (Table 1). The proportions of the major ions in seawater are nearly constant across geographic regions.
How is salinity measured?
Salinity can be measured by several methods. The following methods are arranged in recommended order.
Refractive salinometer: The speed at which light passes through a medium depends on the density of the medium. This principle is used in a refractive salinometer, also called a refractometer, in which drops of sample water are placed on a prism. Since the water sample and the prism have different densities, light that passes through one to the other is refracted at an angle, depending on the density (salinity) of the sample, and is displayed on a scale. Refractive salinometers are relatively accurate, easy to use, and inexpensive.
Hydrometer: The density, or specific gravity, of seawater, depends on salinity; as salinity increases, the specific gravity increases. A hydrometer is commonly used in home aquaria to measure specific gravity. It works on the principle that objects displace a volume equal to their own weight when floating in a liquid (like a ship or iceberg). Therefore, the hydrometer will float higher or lower in the water, depending on the salinity. Charts are required to correct specific gravity readings for temperature and to translate them into salinity (ppt). Hydrometers are inexpensive and easy to use, but are not precise.
Chlorinity: The ratio between chloride content and total salinity is relatively constant in seawater. Therefore, salinity can be calculated from the chloride content, or chlorinity. Chlorinity is measured by titration of seawater against a silver nitrate solution. This method had been used routinely until the 1950s, when simpler methods became more available.
Conductivity: How well a water sample conducts an electrical current is proportional to the concentration of ions in solution. Therefore, salinity can be determined with a conductance-measuring instrument, called a conductivity salinometer or conductivity meter. Since conductivity is a function of temperature, charts or equations are required to convert conductivity (S/cm) readings into salinity (ppt) for a specific temperature.
Dry residue method: Since salinity is the mass of salt per unit mass of water, one could simply evaporate the water and weigh the remaining salts. Unfortunately, accurate measurements are difficult; volatile compounds are lost along with the water, and the remaining residue is hygroscopic, meaning it attracts water, which makes estimates of salinity too high.
Why is salinity variable?
While the ratios between the major ions in seawater are remarkably constant, salinity can vary dramatically from region to region. The salinity in the open ocean is 30 to 35 ppt. The salinities of the Red (40 ppt) and Mediterranean (38 ppt) Seas are higher, while the salinities of the Black (18 ppt) and Baltic (8 ppt) Seas are lower. Estuaries and some coastal waters are considered brackish, with salinity in the range of 0.5 to 29 ppt.
Many factors can increase or decrease salinity. Factors that dilute saltwater and thus decrease salinity include precipitation of rain or snow, river runoff, melting of ice, and groundwater flow. Factors that increase salinity include evaporation and freezing, both of which leave behind the salts, increasing the salinity of the remaining water. For example, some enclosed coastal lagoons or lakes without large freshwater streams or rivers, are excessively saline, with salinity above 40 ppt due to evaporation of water, especially in warm regions of the world.
In areas of Florida where hard clams are aquacultured, rain, river and surface runoff, groundwater flow, and evaporation all have significant impacts on salinity. For example, periods of drought coupled with warm air temperatures and associated evaporation result in coastal salinity that can exceed that of normal ocean water and reach nearly 40ppt. Alternatively, tropical storms and the resulting precipitation can dramatically decrease coastal salinity, to less than 5 ppt. Short-term (hourly) and long-term (daily, monthly, yearly) fluctuations in salinity occur naturally in estuaries and coastal waters. Some areas, such as Charlotte Harbor and the Indian River, are also impacted by the human-controlled release of stored freshwater to the coast.
How does salinity affect the physiology of hard clams?
Unlike fish, hard clams and most marine invertebrates allow the salinity of their blood to vary with the salinity of the external seawater. Therefore, any increase or decrease of the salinity of the surrounding waters will be proportionally reflected in the salinity of the clams' blood. Although hard clams allow the salinity of their blood to vary, they need to keep the concentrations of ions inside their cells relatively constant to maintain the functioning of important metabolic enzymes. The necessity to keep the salinity in cells constant poses some problems, however. When the salinity of saltwater declines, the salinity of the blood will become lower than that of the cells. Water (but not ions) will then move, by the process of osmosis, from the blood (low ion concentration, high water concentration) into the cells (high ion concentration, low water concentration). This will cause the cells to swell, and the extra water will dilute the ions in the cells, disrupting the activity of many metabolic enzymes. Alternatively, when the salinity of the saltwater increases, the salinity of the blood will become greater than that of the cells. As a result, water (but not ions) will move out of the cells by osmosis, the cells will shrink, the cell ions will become more concentrated, and enzyme function will be disrupted.
Hard clams prevent osmosis from occurring and, as a result, maintain cell ion concentrations at desired levels, by regulating the concentrations of free amino acids, which are the building blocks of proteins. When the salinities of the saltwater and blood decline, cells allow free amino acids to leak out, but ions remain in the cell at normal concentrations. The result is that the total concentration of dissolved molecules (ions plus free amino acids) declines along with the external salinity, preventing gain of water by osmosis and swelling of the cells. Similarly, when the salinities of saltwater and blood increase, cells increase their levels of free amino acids so that the total concentration of dissolved molecules within the cells increases. This process prevents the cells from shrinking due to loss of water via osmosis. By regulating free amino acids, cells maintain both their ion concentrations and volumes. This process has limits, however. Although hard clams can tolerate a wider salinity range than many marine mollusks, they cannot tolerate prolonged exposure to either high or low salinity, as discussed below.
What are gross signs of salinity stress?
Clams have the ability to close their valves, or shells, in response to adverse salinity conditions, effectively preventing exposure to the surrounding seawater. Clams can keep their valves closed for several days. During this time they respire anaerobically (without oxygen). Clams subject to salinity stress may exhibit: gaping (a longer-term response), retracted mantle edges (especially in response to abnormally high salinities), or swollen, protruding mantle edges (especially in response to low salinities). Gross signs of adverse environmental conditions in juvenile or adult hard clams may go unnoticed because they are infaunal, living buried in the sediment. However, stressed clams may rise to the surface of the sediment or fail to bury. In laboratory studies, we found that clams held at 25, 35, and 40 ppt actively buried. However, clams did not bury in salinities of 5, 10, or 50 ppt. At 15 and 45 ppt, fewer than 25% of clams had buried after 24 hours. These signs are not necessarily specific indications of salinity stress; they may also be associated with infectious or noninfectious diseases, or other adverse environmental conditions, such as high temperature and low dissolved oxygen concentration.
How does salinity affect hard clam production?
Salinities of 20–30 ppt are considered the optimal physiological range for hard clams. Within this range, pumping rates, feeding rates, growth, and other activities are at their maximum. Although hard clams can adjust to changes in salinity, as described above, there are upper and lower limits to their tolerance. Above and below these limits, the clams will begin to show gross signs of stress. If salinities remain outside these limits for an extended period of time, the clams will not survive.
Our laboratory studies indicated that at spring and fall temperatures of 24°C–26°C (75°F–79°F) growout-size seed clams (10–15 mm shell length) and pasta-size clams (25–30 mm shell length) tolerated salinities between 10 and 40 ppt for longer than 15 days. Both growout-size and pasta-size clams began experiencing increased mortality at approximately 13 days of exposure to 5 ppt, with 54% and 35% mortality, respectively, by the end of 15 days. At 45 ppt, growout-size seed clams began dying by four days, while pasta-size clams began dying at around 8 days of exposure. By the end of 15 days of exposure, these clams experienced approximately 76% (growout-size seed clams) and 44% (pasta-size clams) mortality.
Other environmental conditions affect the ability of clams to survive adverse salinity conditions, including temperature and dissolved oxygen. For example, high temperatures (greater than 32°C or 90°F) and low dissolved oxygen concentrations will intensify the effects of stressful salinity concentrations. Furthermore, physiological condition (energy stores and spawning stage), age, size, and acclimation history also determine the tolerance of a clam to changes in salinity. For example, juvenile clams (seed) are more susceptible to salinity fluctuations than adults. The rate of salinity change is also very important; clams will be more likely to show signs of stress if the salinity changes rapidly (hours to days), for example as the result of a flood event, than if the salinity changes relatively slowly (days to weeks).
How can I manage my crop in response to salinity?
Select a Lease Site Based on Salinity Regime
Salinity plays a crucial role in the growth and survival of clams. Therefore, it is important to take salinity into account when selecting nursery and growout sites. Most successful clam leases have salinities between 20 and 30 ppt the majority of the time. Most sites will experience occasional periods of salinity less than 20 ppt due to river flood stages or heavy rain, which influence the salinity regime of shallow coastal water bodies. Successful sites experience short-lived and infrequent low-salinity events. Not only does salinity impact survival, but repeated periods of decreased salinity will slow growth rates, resulting in a longer growout time to harvest.
Salinity regime also has an effect on the abundance and presence of predators and fouling organisms. For example, fouling of the mesh bags and cover netting by oyster spat is a problem at lower salinities, while tube worms become a problem at higher salinities. To determine the salinity regime of a potential nursery or growout site, monitoring the salinity of the site for one to two years is recommended, or examining any available historical records. Monthly water quality data can be obtained for shellfish harvesting areas in Florida by contacting a Shellfish Environmental Assessment Section (SEAS) field office of the Florida Department of Agriculture and Consumer Services, Division of Aquaculture (see https://www.fdacs.gov/Agriculture-Industry/Aquaculture/Shellfish-Harvesting-Area-Classification). Archived water quality data, collected during 2002–2018 at selected aquaculture lease areas in 6 coastal counties, can be found at http://shellfish.ifas.ufl.edu/water-quality-monitoring.
Understand the Hydrology at Your Site
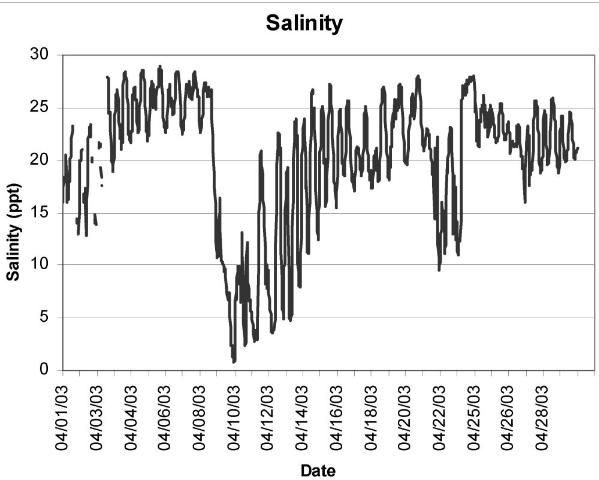
To manage a crop proactively, it is imperative to understand the salinity regime at a given nursery or growout lease. To better understand and respond to seasonal and annual variations in salinity, growers should take frequent salinity measurements, as well as record their activities and subsequent crop survival. Water should be collected near the bottom, not the surface; freshwater is less dense than saltwater and will therefore float on top of the saltier water. Stratification of the water column can occur during rain or flood events, resulting in erroneously low salinity measurements. We know, for example, that the Suwannee River Sound is highly productive, resulting in rapid clam growth rates. However, we also know that the Suwannee River and other Florida rivers regularly experience spring flooding events (freshets), which dramatically lower salinity over a short period of time (Figure 1). Therefore, following heavy rain storms in the watershed, or during El Niño weather phenomena, it would be prudent to view real-time river gauge data, available from the National Weather Service (http://www.weather.gov/) and the US Geological Survey (http://waterdata.usgs.gov/fl/nwis/), to determine if a low-salinity event is imminent. If so, planting of seed, for example, should be delayed, or an alternate site could be selected. If a grower leases multiple sites, one that is least likely to experience freshets could be reserved as a field nursery site and hardier, larger growout-size seed clams could be planted at sites that are more likely to experience fluctuations in salinity.
Buy Nurse and Plant Seed at Compatible Salinities
Nursery seed clams of 1 to 6 mm in shell length are more vulnerable to extremes and changes in salinity than are growout-size seed clams (10–15 mm shell length). Therefore, particular care should be taken with these smaller clams. For example, when purchasing seed, the salinity profiles of the seed source location and the land-based or field-based nursery sites should be examined. For optimal survival, the difference in salinity between the two sites should be less than 5 ppt. This is especially true in the summer when the added stressor of high temperature may intensify the effects of salinity on the physiology of the clams. If the seed source and nursery planting sites differ excessively in salinity, the grower has several options. Growers might wait to purchase or pick up seed until the salinity at the two sites are more similar, they might request that the seed supplier acclimate the clams to a salinity more similar to that of the nursery planting site, or they might decide to locate an alternate supplier whose seed is already acclimated to a salinity similar to that of the planting site.
Conduct Activities at Salinities of 20 ppt or more
Salinities of less than 20 ppt cause physiological stress and changes in clam behavior. Some farm activities may further intensify stress and increase the risk of mortality. For example, clams that are either planted or transferred to growout bags during low-salinity events do not bury as quickly as they would at higher salinities, leaving them more vulnerable to predation and fouling. In addition, clams harvested at low salinities may have a shorter shelf life in refrigerated storage. It is prudent to delay planting, transferring, and harvesting, until salinities have returned to normal.
Anticipate Extreme Low Salinity Events
Hurricanes, storms, and flood-control measures can rapidly reduce coastal salinities to levels that are incompatible with clam survival: river gauge data, http://www.weather.gov, streamflow data http://waterdata.usgs.gov/fl/nwis/current?type=flow&group_key=county_cd&search_site_no_station_nm, and flood control data available from the US Army Corp of Engineers (http://www.saj.usace.army.mil/Missions/EmergencyOperations/ProgramsPolicies.aspx [29 October 2012]) may be consulted to determine if an extreme event is likely to occur. If it is determined that such an event is likely, several measures could be taken to reduce catastrophic crop loss. For example, clams that have reached a marketable size could be harvested and processed prior to the event, reducing the risk of losing this valuable portion of the total crop.
Seed clams in land-based nurseries are also at risk during low salinity events since water is drawn into the raceways or weller systems directly from an estuary. While moving field nursery bags and larger clams in growout bags to a more favorable site presents logistical problems, growers may consider relocating land-based nursery clams, which are more vulnerable to salinity extremes and are easier to move. Seed can be held in a cooler (65°F–70°F) for 1–2 days before needing to be immersed ("given a drink") in saltwater. Alternatively, growers may elect to maintain the seed in nursery raceways or weller systems by "salting" the tanks. The addition of a brine solution to static raceways or weller systems will adequately maintain nursed seed for several days, or longer if aeration is incorporated. In the event of a power outage, battery-operated aerators or air pumps may be used to maintain adequate oxygen levels in the tanks.
Salting Land-Based Nursery Tanks
Bulk rock salt is relatively inexpensive and is available as water-softening salt from home improvement stores. Be sure to use salt that is primarily sodium chloride (NaCl). Nursery operators might elect to keep on hand enough salt to raise the salinity in all their tanks or raceways to 15–20 ppt. Table 2 and Table 3 provide estimates of the amount of rock salt required to maintain salinity in two types of tanks. To determine the amount of salt to use per raceway or weller system, the salinity of the water should be measured and the target salinity determined. For example, if the current salinity in a raceway is 5 ppt, and the operator wants to raise the salinity to 20 ppt, the salt amounts in the 15 ppt column of Tables 2 or 3 would be added. Rather than adding the salt directly to the raceway or weller system, a concentrated brine solution should be made in a separate container with an amount of water sufficient to completely dissolve the salt (see volumes in parentheses in Table 2 or Table 3). The salt will dissolve faster if the container is agitated. Once the salt is fully dissolved, the brine solution can be slowly mixed into the raceway or weller system. Salinity should be measured frequently to determine if the target salinity has been reached and to avoid over-salting the system due to miscalculations. If your tank sizes differ from those shown in Table 2 or Table 3, refer to Box 2.
Summary
Salinity in clam leases is an environmental factor that strongly affects clam survival and growth. Clam growers cannot control salinity on their leases, so they must develop management strategies that adapt to it. The essential first step is salinity monitoring; with this information the clam grower can evaluate lease quality, determine optimal seed clam nursery areas, and react to extreme salinity events. Salinity in a land-based nursery can be adjusted temporarily, if the clam grower is prepared. Again, salinity monitoring is the essential first step. In order to minimize the potential economic impact to the industry, it is prudent to be aware of environmental conditions and be prepared to properly assess any instances of mortality. Assistance from UF/IFAS Extension shellfish specialists is available.
Leslie Sturmer
UF/IFAS Extension Shellfish Agent
UF/IFAS Extension
Cedar Key, FL 32625
Phone: 352-543-5057
E-mail: LNST@ufl.edu
Shirley Baker
UF/IFAS School of Forest, Fisheries, and Geomatics Sciences
Fisheries and Aquatic Sciences Program
7922 NW 71 st St
Phone: 352-273-3627
E-mail: sbaker25@ufl.edu
Glossary of Terms Used
Acclimation—The gradual and reversible adjustment of physiological processes in response to changing environmental conditions
Anaerobic respiration—The oxidation of molecules to produce energy in the absence of oxygen
Brackish water—Diluted saline water (between 0.5 and 29 ppt salt), which occurs in regions where ocean waters mix with freshwater sources
Chloride—One of the major anions (net negative charge) commonly found in water
Concentration—The amount of a substance dissolved in a given amount of liquid
Conductance—The ability of a solution to carry an electrical current
Density—A measure of mass per unit volume
Downweller—An open-ended cylinder in which clam seed are suspended on a screen and water flows down over the animals
Free amino acids—Small molecules that are the basic building blocks of proteins; not currently linked to form proteins
Groundwater—Water underground in an aquifer or water table
Growout-size clam seed—Refers to clams greater than 10 mm in shell-length that are grown on open-water leases in large-mesh bags
Hygroscopic—Describes a substance that readily attracts and retains water
Infaunal—Aquatic animals that live in the substrate, usually a soft sediment
Ion—An atom or molecule that carries a positive or negative electrical charge, such as Na+ or Cl-
Larva—Immature stage of an animal that differs markedly in structure from the adult
Mantle—Folds of the body wall that form a skirt around the body of a mollusk, terminating in an edge, the mantle margin
Metabolic enzymes—Protein substances that catalyze (cause or speed up) chemical reactions involved in processes such as the breakdown of food, or production of energy.
Metamorphosis—The marked and rapid transformation of a larva into an adult form
Osmosis—The passage of water from a weak solution of low concentration to a more concentrated solution across a semi-permeable membrane that allows passage of the water but not the dissolved substances
Phytoplankton—Freely floating microscopic aquatic plants (algae)
Protein—Large complex molecules made up of chains of amino acids, serving as enzymes, hormones, and structural components of cells
Raceway—Shallow tank or tray with horizontal flow of seawater
Refractometer—An instrument that measures the bending of light (refraction) through a liquid; it can be used to measure the salinity of water
Salinity—The concentration of salts dissolved in water
Salinometer—An instrument for measuring salinity using electrical conductivity
Seed—Refers to clams less than 10 mm in shell length
Signs—Objective evidence of disease
Specific gravity—The weight of a solution compared to the weight of an equal volume of pure water
Solute—A substance dissolved in a solvent, thus forming a solution
Solution—A homogeneous mixture of one or more substances (solutes) dissolved in another substance (solvent)
Solvent—A substance, usually a liquid, capable of dissolving other substances
Titration—A chemical technique used to determine the concentration of a solute in a solution by drop wise addition of an indicator chemical to the sample until a color change takes place
Upweller—An open-ended cylinder in which clam seed are suspended on a screen and water flows up between the animals
Volatile compound—A substance that readily evaporates at room temperature
Weller system—Consists of open-ended cylinders suspended in a water reservoir or tank. Seawater circulates among the seed clams (either up or down), which are supported on a screen at the bottom of the cylinder.
Further Reading
Baker, S. M., P. Baker, D. Heuberger, and L. Sturmer. 2005. "Short-term effects of rapid salinity reduction on seed clams (Mercenaria mercenaria)." J. Shellfish Res. 24: 29–33.
Berger, V. J., and A. D. Kharazova. 1997. "Mechanisms of salinity adaptations in marine molluscs." Hydrobiologia 355: 115–126.
Castagna, M. and P. Chanley. 1973. "Salinity tolerance of some marine bivalves from inshore estuarine environments in Virginia waters on the western mid-Atlantic coast." Malacologia 12: 47–96.
Chanley, P. E. 1957. "Survival of some juvenile bivalves in water of low salinity." Proc. Natl. Shellfish. Assoc. 48: 52–65.
Goldburg, R. and G. H. Wikfors. 1992. "Growth of hard clams in Long Island Sound: sorting out the determining factors." Environ. Manag. 16: 521–529.
Kraeuter, J. N. and M. Castagna. 2001. Biology of the Hard Clam. Elsevier, Amsterdam, The Netherlands. 751 pp.
Malouf, R. E. and V. M. Bricelj. 1989. Comparative biology of clams: environmental tolerances, feeding, and growth. Pages 23–71, In: J. J. Manzi and M. Castagna (Eds.), Clam Mariculture in North America. Elsevier, Amsterdam.
Pratt, D. M., and D. A. Cambell. 1956. "Environmental factors affecting growth in Venus mercenaria. Limnol." Oceanogr. 1: 2–17.
Rice, M. A. and J. A. Pechenik. 1992. "A review of the factors influencing the growth of the northern quahog, Mercenaria mercenaria (Linnaeus, 1758)." J. Shellfish. Res. 11: 279–287.
Roegner, G. C. and R. Mann. 1991. Hard clam, Mercenaria mercenaria. Pages 5.1–5.17, In: S. L. Funderburk, J. A. Mihursky, S. J. Jordan, and D. Riley (Eds). Habitat requirements for Chesapeake Bay Living Resources. 2nd ed. Solomons, MD: Chesapeake Research Consortium.
Schmidt-Nielsen, K. 1997. Animal Physiology; Adaptation and Environment. 5th ed. New York, NY: Cambridge University Press.
Sturmer, L. N. 2004. Florida Shellfish Aquaculture Extension. http://shellfish.ifas.ufl.edu/
Wells, H. W. 1957. "Abundance of the hard clam Mercenaria mercenaria in relation to environmental factors." Ecology 38: 123–128.
English units: Weight of salt (NaCl-Sodium Chloride) required to raise salinity by 5, 10, 15, or 20 ppt. Numbers in parentheses represent the minimum volume of water required to dissolve the given weight of salt. The salt will dissolve faster in greater volumes of water.
Metric units: Weight of salt (NaCl-Sodium Chloride) required to raise salinity by 5, 10, 15, or 20 ppt. Numbers in parentheses represent the minimum volume of water required to dissolve the given weight of salt. The salt will dissolve faster in greater volumes of water.