What are the megalocytiviruses?
The megalocytiviruses are an important group (genus) of fish viruses in the family Iridoviridae (the iridoviruses). Megalocytiviruses cause systemic infections that can result in moderate to heavy losses in many different species of freshwater and marine fishes in both cultured and wild stocks. In some disease outbreaks, 100% losses have occurred in under one week. Megalocytiviruses have been reported in fish in the United States as well as other parts of the world, especially Asia.
Currently, nearly all isolates from diseased fish appear to be strains of the same virus species. Isolates have been divided into two species: 1) Infectious spleen and kidney necrosis virus (ISKNV) and 2) Scale drop disease virus. The first species (Infectious spleen and kidney necrosis virus) includes three major genotypes based on their genetic similarities and differences: a) infectious spleen and kidney necrosis virus (ISKNV); b) red sea bream iridovirus (RSIV); and c) turbot reddish body iridovirus (TRBIV). The fact that one genotype has the same name as the species (i.e., both are ISKNV) is confusing, so when discussing ISKNV, it should be clarified whether one is referring to the species or the genotype.
Strains closely resembling ISKNV have been reported to cause disease in numerous species of ornamental freshwater and marine fishes. In the early 1990s, RSIV was first observed in Japan and since then has been reported primarily in Asian marine finfish. Today, RSIV is reportable to the World Organization for Animal Health (OIE) and the United States Department of Agriculture-Animal and Plant Health Inspection Service (USDA-APHIS). The third subgroup TRBIV has been reported predominantly in Asian flounder species. New viral isolates from other fish species are currently being evaluated by scientists to determine their relationships to these three main groups. This publication provides disease, diagnostic, and management information on megalocytiviruses in fish for producers, wholesalers, retailers, and others who work with fish and may be unaware of this disease.
Which fish species are susceptible?
Ornamental finfish species known to be susceptible to megalocytiviruses are listed in Table 1. Many popular aquarium fish are on this list, including freshwater angelfish (Pterophyllum scalare), other cichlids (Family Cichlidae), swordtails, sailfin mollies, and other common live-bearers (Family Poeciliidae) and a number of species of gourami (Family Osphronemidae and Family Helostomatidae).
Numerous marine and freshwater food and game finfish species have also been shown to be susceptible to infection by megalocytiviruses including: jacks and pompanos (several species, Family Carangidae), mackerels and tuna (several species, Family Scombridae), grouper (several species, Family Serranidae), cobia (Rachycentron canadum), largemouth bass (Micropterus salmoides), barramundi (Lates calcarifer), redfish (Sciaenops ocellatus), hybrid striped bass (Morone saxatilis × M. chrysops), and gray mullet (Mugil cephalus). A more complete list is available online at OIE's website https://www.woah.org/fileadmin/Home/eng/Health_standards/aahm/current/2.3.07_RSIVD.pdf.
The first megalocytivirus discovered in a wild North American fish was recently described from the threespine stickleback (Gasterosteus aculeatus, Family Gasterosteidae) in Canada, where outbreaks in 2007–2008 resulted in low mortality. DNA studies demonstrate that the cause of this outbreak is yet another unique strain of the megalocytivirus (Waltzek et al. 2010), and is not one of the three types mentioned above.
Within what temperature range can megalocytiviruses infect fish and cause disease?
Megalocytiviruses have been reported to cause disease at water temperatures ranging from 20–32°C (68–89.6°F).
What are typical external and internal signs of disease?
Fish infected with megalocytivirus develop clinical signs of disease that are non-specific, meaning that they are similar to clinical signs observed with many other diseases. These include lethargy, loss of appetite, darkening, abnormal swimming (including spinning) or position in the water, increased respiration, distended body cavity (coelomic distension), ulceration, hemorrhages (including pinpoint hemorrhages on the skin and gills), pale gills/anemia, fin erosion, white feces, and heavy mortalities.
Although studies are ongoing, one research group has suggested that the virus may target and be spread by white blood cells or other immune cells in the body (Lee et al. 2009). This may explain why the virus can be found throughout the body in severe cases. During necropsy, megalocytovirus-infected fish are often observed to have damage to many internal organs (i.e., tissue death, necrosis) especially the spleen (which is often enlarged), kidney, and liver. Other organs and tissues, including muscles, gonads, heart, gills, and the gastrointestinal tract, may also be affected. Some fish may have amber or hemorrhagic fluid visible within the body cavity.
Concurrent (i.e., occurring at the same time) infections with bacteria, parasites, or fungi may result in additional disease signs and complicate the diagnostic process. Unfortunately, most sick fish often have more than one disease contributing to their conditions. Determining the presence or absence of megalocytovirus in a group of diseased fish is important when making decisions about the disposition of the entire exposed population.
How do I tell if my fish has a megalocytivirus infection?
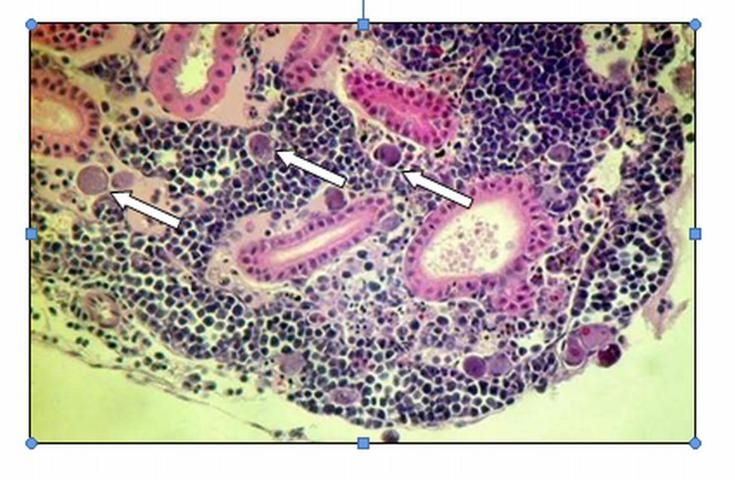
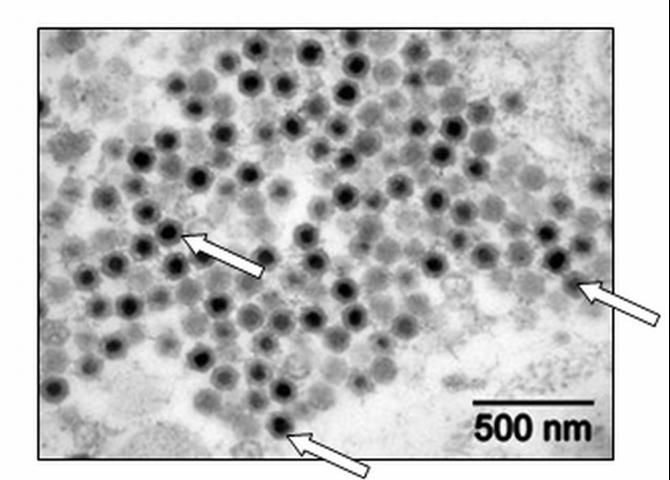
Special diagnostic tests are required to confirm a diagnosis of megalocytivirus in fish. Only a fish health professional or fish disease diagnostic laboratory has the equipment and expertise to make this determination. Appropriate diagnostic tests include histopathology, in which a preparation of thin fixed tissue sections are placed on a glass slide, stained, and examined under the microscope (Figure 1); virus isolation, which requires growing the virus in cell culture; testing for evidence of antibodies made by the fish against the virus; use of an electron microscope to look for viral particles themselves (Figure 2); and use of polymerase chain reactions (PCR) or DNA probes (pieces of genetic material that will bind specifically to megalocytiviruses), in order to reveal evidence of viral DNA. Because these are highly specialized tests, not all laboratories are equipped to run them. Contact your local fish health professional for diagnostic assistance.
Credit: Roy P. E. Yanong
Credit: Roy P. E. Yanong
When a fish health professional or pathologist examines tissues prepared for histopathology, the most distinctive finding is the presence of large, bright blue (basophilic) or pale, foamy cells (Figure 1). These enlarged, infected cells have become virus factories and are typically found in the spleen, kidney, or liver. Other associated pathology, including dead tissue (necrosis), can be found in just about any organ.
A disease outbreak is rarely due to the simple presence of a pathogen. Many different factors often contribute, and therefore a complete review of the facility including water quality records, movement of animals in or out, and a review of husbandry procedures (including nutrition, stocking rates, cleaning and disinfection, and other related biosecurity and system management protocols), be carried out as part of a disease investigation. A thorough necropsy carried out by a fish health professional of representative fish showing clinical signs of disease should be performed.
Can megalocytivirus infections be confused with any other diseases?
Yes. Megalocytivirus infections in fish can cause disease signs that are similar to many other infectious diseases, especially those caused by bacteria or other viruses. They may also resemble disease signs caused by environmental toxins or poor water quality. A thorough fish disease investigation is required to confirm the presence of megalocytovirus infection and determine whether other factors are contributing to the disease outbreak.
What factors contribute to development and spread of megalocytivirus disease?
As with other viral diseases, environmental and procedural stressors will weaken the immune systems of fish and therefore can increase susceptibility to infectious diseases including megalocytivirus. Good husbandry may help reduce infection rates and losses (for Florida producers, see "Aquaculture Best Management Practices" available at: https://www.fdacs.gov/Agriculture-Industry/Aquaculture/Aquaculture-Best-Management-Practices). Warmer temperatures appear to facilitate infection and disease since many past cases seen in Florida occurred during the heat of the summer and early fall or after temperature spikes occurred in indoor recirculating systems (Yanong and Terrell 2003).
Laboratory studies have demonstrated that megalocytiviruses can be spread by cohabitation (from fish to fish in the same water body) or by exposure to virus-contaminated water. It is probable that megalocytiviruses may also be spread by consumption of infected fish or tissues and by use of virus-contaminated equipment.
Current studies suggest that megalocytiviruses have the ability to infect numerous species of freshwater and marine fish. Spread of the disease, therefore, may occur by introduction of infected fish into naïve populations (groups that have not been exposed to a disease before are called "naïve"). Infectivity may be increased if the immune systems of naïve fish are compromised due to environmental or other stressors, including recent shipping or handling.
Transmission of megalocytivirus from infected broodstock to eggs and sperm (i.e., vertical transmission) has not been proven to date.
Do all fish that get infected with megalocytivirus die?
No. Although, as described previously, megalocytivirus infections have been associated with significant mortalities within populations—sometimes as high as 100%—low-level infections have been seen with lower mortality rates in some populations (Yanong, unpublished data). Several studies using highly sensitive molecular detection methods have demonstrated that clinically healthy fish may test positive for megalocytivirus. It is unknown whether these fish can serve as reservoirs for future disease outbreaks.
Can you treat fish infected with megalocytivirus?
Currently there are no effective treatments for most viral infections of fish, including those caused by megalocytivirus. Depopulation followed by disinfection is recommended.
What can I do to help prevent a megalocytivirus outbreak?
Good husbandry and biosecurity are important, as is establishment of a good working relationship with a fish health professional and/or diagnostic laboratory. If you suspect you may have fish with a megalocytivirus infection, sampling and special tests will be necessary. Contact a fish health professional for assistance.
Ideally, producers should quarantine any new fish in a separate building or area and follow appropriate biosecurity protocols (have dedicated equipment and use appropriate disinfectants) before adding the new fish to existing farm populations. If possible, producers should also submit a representative sample of fish for additional testing, especially if any appear diseased or if mortalities occur. Although transmission from broodstock to offspring has not been proven to date, producers should also consider physical separation of broodstock from other life stages (e.g., separate building, separate equipment, separate ponds, appropriate disinfection measures between such areas) as a general, good biosecurity practice.
Wholesalers and retailers should consider separating incoming fish groups by origin (e.g., imported vs. domestic, or by country of origin) and species to prevent or reduce likelihood of spread. Because megalocytiviruses can be spread by contaminated water, efforts should be made to use separate equipment (nets, siphon hoses) for each system, and to disinfect items between uses. Judicious placement of footbaths and hand disinfectants (alcohol spray or hand washing stations) may be warranted. Sick fish should be isolated if possible and handled separately (see UF Extension publication Fish Health Management Considerations in Recirculating Aquaculture Systems – Part 1: Introduction and General Principles. Circular FA-120: https://edis.ifas.ufl.edu/fa099).
Use of a water source that may have contained other fish or other potential sources of pathogens (e.g., a creek, lake, or other "unprotected" water source) will increase risk as well. It is safer to use water from "protected" water sources, such as deep wells, or water that has been processed with adequate ultraviolet (UV) sterilization or chlorinated and dechlorinated (see below).
Vaccines have helped reduce disease outbreaks and mortalities for many other different finfish diseases, and preliminary research outside the United States indicates they may work for this disease as well. However, no commercial vaccines are currently available in the United States.
If I want to disinfect equipment after handling infected fish, what can I use?
Experimental studies have shown that iridoviruses on equipment, tanks, and other surfaces can be inactivated in several different ways. Equipment and surfaces can be sterilized of iridoviruses with high temperatures alone (50°C [122°F] or greater for 30 min). Alternately, equipment and surfaces can be sterilized of iridoviruses with any of the following chemical treatments if performed at 25°C (77°F) for a period of 15 minutes: potassium permanganate (100 mg/L or higher), formalin (2000 mg/L or higher), or 5% sodium hypochlorite (liquid bleach) at 200 mg/L or higher. Raising the pH to 11 or greater for 30 minutes (or more) may also be effective and may be a method for disinfection of earthen ponds (He et al. 2002). Use of UV sterilization also has been shown to be fairly effective against iridoviruses. When red sea bream iridovirus-contaminated water was exposed to UV sterilization, doses ranging from 1,000 to 3,000 μW•sec/cm2 resulted in 99% or more decrease in infectivity (Kasai et al. 2002).
Summary
The megalocytiviruses are an important group of viruses that infect many different species of fish. Infections can result in significant (up to 100%) mortalities, and some strains appear to have the ability to infect numerous species. The disease can be spread from fish to fish by contaminated water and most likely also by ingestion of infected tissues. Thermal stress may promote outbreaks.
Because clinical signs of disease are non-specific and are similar to those seen with other infectious diseases, specialized viral diagnostic tests are required to confirm a diagnosis of megalocytovirus.
Quarantine and test a representative sample from a new group of fish, including future broodstock before mixing with established fish populations on the farm. Submit any diseased fish for diagnostic evaluation.
There are no good methods for treatment of infected fish, and depopulation may be the best option. Contain dead and depopulated fish and dispose of them appropriately to minimize the potential for spread. There are a number of good options for disinfection of equipment and tanks, including use of sodium hypochlorite (bleach) at 200 mg/L or higher or potassium permanganate at 100 mg/L or higher.
Finally, work with a fish health professional for an accurate diagnosis of any disease problems, including suspected megalocytivirus infections, and for recommendations on management and prevention. Maintaining a good working relationship with a fish health professional is recommended for all businesses raising or selling live aquatic products.
References and Suggested Reading
Anderson, I., H. Prior, B. Rodwell, and H. Go. 1993. Iridovirus-like virions in imported dwarf gourami (Colisa lalia) with systemic amoebiasis. Australian Veterinary Journal 70:66–67.
Armstrong R. D. and H. W. Ferguson. 1989. Systemic viral disease of the chromide cichlid Etroplus maculatus. Diseases of Aquatic Organisms 7:155–157.
Bovo, G. and D. Florio. 2008. Viral diseases of cultured marine fish. Pages 202–216 in Eiras, J.C., H. Segner, T. Wahli, and B. G. Kapoor, editors. Fish Diseases, Volume 1. Science Publishers, Enfield, NH.
Division of Aquaculture, Florida Department of Agriculture and Consumer Services. Aquaculture Best Management Practices. Available: https://www.fdacs.gov/Agriculture-Industry/Aquaculture/Aquaculture-Best-Management-Practices (Accessed February 5, 2024).
Fraser W. A., T. J. Keefe, and B. Bolon. 1993. Isolation of an iridovirus from farm-raised gouramis (Trichogaster trichopterus) with fatal disease. Journal of Veterinary Diagnostic Investigation 5:250–253.
Gibson-Kueh, S., P. Netto, G. H. Ngoh-Lim, S. F. Chang, L. L. Ho, Q. W. Qin, F. H. Chua, M. L. Ng, and H. W. Ferguson. 2003. The pathology of systemic iridoviral disease in fish. Journal of Comparative Pathology 129:111–119.
Go, J., M. Lancaster, K. Deece, O. Dhungyel, and R. Whittington. 2006. The molecular epidemiology of iridovirus in Murray cod (Maccullochella peelii peelii) and dwarf gourami (Colisa lalia) from distant biogeographical regions suggests a link between trade in ornamental fish and emerging iridoviral diseases. Molecular and Cellular Probes 20:212–222.
Go, J. and R. Whittington. 2006. Experimental transmission and virulence of a megalocytivirus (Family Iridoviridae) of dwarf gourami (Colisa lalia) from Asia in Murray cod (Maccullochella peelii peelii) in Australia. Aquaculture 258:140–149.
He, J. G., K. Zeng, S. P. Weng, and S. M. Chan. 2002. Experimental transmission, pathogenicity, and physical-chemical properties of infectious spleen and kidney necrosis virus (ISKNV). Aquaculture 204:11–24.
Jeong, J. B., H. Y. Kim, L. J. Jun, J. H. Lyu, N. G. Park, J. K. Kim, and H. D. Jeong. 2008. Outbreaks and risks of infectious spleen and kidney necrosis virus disease in freshwater ornamental fishes. Diseases of Aquatic Organisms 78:209–215.
Kasai, H., M. Yoshimizu, and Y. Ezura. 2002. Disinfection of water for aquaculture. Proceedings of International Commemorative Symposium 70th Anniversary of the Japanese Society of Fisheries Science 68 (Supplement 1): 821–824.
Kawakami H. and K. Nakajima. 2002. Cultured fish species affected by red sea bream iridoviral disease from 1996 to 2000. Fish Pathology 37:45–47.
Kim, W., M. Oh, J. Kim, D. Kim, C. Jeon, J. Kim. 2010. Detection of megalocytivirus from imported tropical ornamental fish, paradise fish Macropodus opercularis. Diseases of Aquatic Organisms 90:235–239.
Koda, S.A., Subramaniam, K., Pouder, D.B. et al. Complete genome sequences of infectious spleen and kidney necrosis virus isolated from farmed albino rainbow sharks Epalzeorhynchos frenatum in the United States. Virus Genes 57, 448–452 (2021).
Lee, N. S., J. W. Do, J. W. Park, and Y. C. Kim. 2009. Characterization of virus distribution in rock bream (Oplegnathus fasciatus; Temminck and Schlegel) infected with megalocytivirus. Journal of Comparative Pathology 141:63–69.
Leibovitz, L. and R. C. Riis. 1980. A viral disease of aquarium fish. Journal of the American Veterinary Medical Association 177:414–417.
Paperna, I., M. Vilenkin, and A. P. A. de Matos. 2001. Iridovirus infections in farm-reared tropical ornamental fish. Diseases of Aquatic Organisms 48:17–25.
Petty, B. D. and W. Fraser. 2005. Viruses of pet fish. Veterinary Clinics of North America: Exotic Animal Practice, Special Issue: Virology 8(1):67–84.
Schuh, J. C. and I. G. Shirley. 1990. Viral hematopoietic necrosis in an angelfish (Pterophyllum scalare). Journal of Wildlife Medicine 21:95–98.
Sudthongkong, C., M. Miyata, and T. Miyazaki. 2002a. Iridovirus disease in two ornamental tropical fishes: African lampeye and dwarf gourami. Diseases of Aquatic Organisms 48:163–173.
Sudthongkong, C., M. Miyata, and T. Miyazaki. 2002b. Viral DNA sequences of genes encoding the ATPase and the major capsid protein of tropical iridovirus isolates which are pathogenic to fishes in Japan, South China Sea and Southeast Asian countries. Archives of Virology 147:2089–2109.
Waltzek, T. B., G. D. Marty, M. E. Alfaro, W. R. Bennett, M. Haulena, E. S. Weber, and R. P. Hedrick. 2010. Smoldering mortality in a captive population of threespine stickleback (Gasterosteus aculeatus). Proceedings of the 35th Eastern Fish Health Workshop, Shepherdstown, West Virginia.
Wang, Y. Q., L. Lü, S. P. Weng, J. N. Huang, S. M. Chan., and J. G. He. 2007. Molecular epidemiology and phylogenetic analysis of a marine fish infectious spleen and kidney necrosis virus-like (ISKNV-like) virus. Archives of Virology 152:763–773.
Weber, E. S., T. B. Waltzek, D. A. Young, E. L. Twitchell, A. E. Gates, A. Vagelli, G. R. Risatti, R. P. Hedrick, and S. Frasca. 2009. Systemic iridovirus infection in the Banggai cardinalfish (Pterapogon kauderni Koumans 1933). Journal of Veterinary Diagnostic Investigation 21:306–320.
World Organisation for Animal Health (OIE). 2010. Manual of Diagnostic Tests for Aquatic Animals 2010. World Organisation for Aquatic Animal Health, Paris. http://www.oie.int/index.php?id=606&L=Osummry.htm. (November 2011).
Yanong, R .P. E. 2009. Fish Health Management Considerations in Recirculating Aquaculture Systems –Part 1: Introduction and General Principles. University of Florida, UF IFAS Cooperative Extension Service, Circular FA-120. Available: https://edis.ifas.ufl.edu/fa099. (November 2010).
Yanong, R. P. E. 2009. Fish Health Management Considerations in Recirculating Aquaculture Systems – Part 2: Pathogens. University of Florida, UF IFAS Cooperative Extension Service, Circular FA-121. Available: https://edis.ifas.ufl.edu/fa100 (November 2010).
Yanong, R. P. E. 2009. Fish health management considerations in recirculating aquaculture systems – part 3: general recommendations and problem-solving approaches. University of Florida, UF IFAS Cooperative Extension Service, Circular FA-120. Available: https://edis.ifas.ufl.edu/fa101. (November 2010).
Yanong, R. P. E. and S. P. Terrell. 2003. Iridoviral-associated disease in oscars (Astronotus ocellatus). Proceedings of the 34th Annual Conference of the International Association for Aquatic Animal Medicine, Waikoloa, Hawaii.