Introduction
Peach cobbler, peach ice cream, and ripe peaches picked from the tree are some of the ways that we enjoy peaches. Organic peach acreage in the United States increased more than any other type of organic fruit between 2008 and 2011, but that increase occurred west of the Rocky Mountains (Perez and Plattner 2013). In 2019, organic peaches were produced on 238 farms representing over 3,237 acres. These farms, primarily located in California, Washington, Colorado, and Oregon, grew 20,208 tons of peaches worth over $36 million (USDA NASS 2020). In the southeastern United States, the majority of peach production occurs in South Carolina (17,566 acres) and Georgia (11,877 acres), but only three farms in these states were certified organic (organic certified acreage data not published; USDA NASS 2020).
Approximately 2,000 acres of peaches are grown in Florida, mostly in Martin, St. Lucie, and Polk Counties, but only four farms (24 acres) are USDA organic certified (USDA NASS 2020). The combination of increased consumer demand for organic products and limited organic peach production in the Southeast may indicate that market opportunities exist for expanded organic production in Florida. In general, organic farmers in Florida are optimistic, and results from a recent survey of all USDA-certified organic farms in Florida revealed that 30% of the operations planned to increase production, while only 4% planned to discontinue production (USDA NASS 2020). Organic peach production in Florida can be accomplished with proper management, including cultivar selection, pruning, thinning, fertilization, irrigation, integrated pest management, harvesting, and postharvest handling (Figure 1).
This publication is designed for small- and mid-size farm operators who are certified organic or who desire to use organic practices, and for producers selling fruit directly to consumers. This fact sheet includes recommendations for best practices and references for different types of inputs. This document also references other UF/IFAS Extension EDIS publications that may mention conventional inputs that would be prohibited in organic systems; please verify compliance with your certification agency before using any inputs on your farm.

Credit: David Campbell, UF/IFAS
Market Opportunities
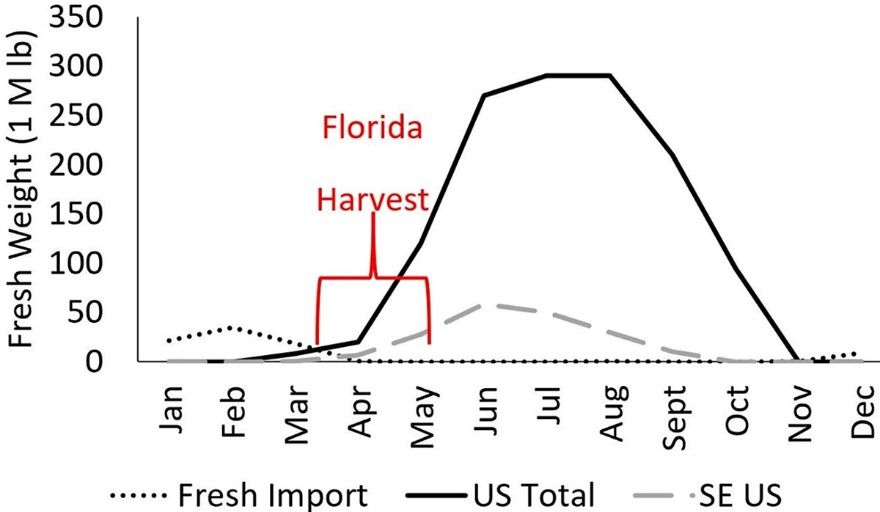
The first domestically grown peaches are harvested as early as March in south-central Florida. In May peaches become available from Georgia, South Carolina, and California, with peak domestic production in June–August (Figure 2). In 2019, the farm-gate price for all peaches sold in May was worth 46% more than for peaches sold in June (USDA ERS 2019). In Florida, the combination of a price-premium for both early and organic fruit is offset by small fruit size as compared to the industry standard minimum 2.5 inches (6.4 cm) in diameter (Figure 3). Due to small fruit size and limited production, most Florida-grown organic peaches are sold from farm stands and pick-your-own (U-Pick) operations.
Credit: undefined
Growers selling directly to the public may utilize roadside stands, farmers’ markets, or community-supported agriculture models. Growers could also attract customers directly to the farm through U-Pick operations or agritainment, including agrotourism and farm entertainment. For more information on the organic industry in Florida and these other direct-to-consumer sales activities, visit the EDIS publications FE732, Economics of the Organic Food Industry in Florida (https://edis.ifas.ufl.edu/fe732), RM008, Agritainment: A Viable Option for Florida Producers (https://edis.ifas.ufl.edu/rm008), and AEC623, Florida’s Agritourism Laws (https://edis.ifas.ufl.edu/wc285).
Production
Planting Stock
If you are a producer starting a new orchard or if you plan to transition some conventional acreage to organic, keep in mind that your soil and nursery stock must be managed using approved methods. Maintain the following thinking as you develop your organic system plan: the system is certified; the inputs are compliant or approved for use in that system; and the crop bears the organic label.
Whether the land is owned or leased, producers seeking certification should be ready to provide 3 years of field management records to their certification agency. Complete and accurate records are necessary, even if the field hasn’t been planted to a crop. The land must not have received prohibited substances for a minimum of three years before the expected harvest date in order for the land to be eligible for certification. Although more common for blueberry (Phillips et al. 2020), some producers may grow peach trees in pots that are arranged on landscape fabric or under a protective structure. In a 2019 policy memo, the USDA NOP deputy administrator clarified that land used for container systems as well as the container system itself must meet the NOP requirements (Tucker 2019). In other words, the growing medium, stock plants, inputs, as well as the soilunder the pots must meet the standards for certification to sell the fruit with a certified organic label. This rule was added to ensure organic integrity.
As of 2020, all commercially available cultivars in Florida are grafted on the ‘Flordaguard’ rootstock (Sherman et al. 1991), which provides resistance to the endemic root-knot nematode, Meloidogyne floridensis (Handoo et al. 2004), and is productive in Florida’s limited chill hour conditions. Peach cultivars are planted on other rootstocks that are suitable for different regions and growers are encouraged to confirm the scion-rootstock combination before purchase. Peach trees have an estimated life span of 10–15 years, and it is recommended that growers include replanting strategies in their organic systems plan. For example, if trees are purchased from conventional nurseries, certified organic growers will need to wait at least 12 months before marketing the fruit as organic even if the tree bears fruit before the end of the required waiting period (Table 1). Your local UF/IFAS Extension agent will be able to assist you with locating nurseries in your area (https://sfyl.ifas.ufl.edu/find-your-local-office/).
Trees from nurseries are generally not pruned but should be pruned during the next dormant season (see training and pruning section below and EDIS publication HS1111, Training and Pruning Florida Peaches, Nectarines, and Plums [https://edis.ifas.ufl.edu/hs365] for more information). Trees to be planted in the fall should be approximately 2.5 to 4 feet (76 to 122 cm) tall, be generally healthy with green leaves, and have no damage or visible gum on the trunk (see insect pest and pathogen management section below). Between 117 and 290 trees can be planted per acre with 10 to 15 feet (3 to 4.5 meters) between trees (within row) that are grown in rows that are 15 to 25 feet (4.5 to 7.6 meters) apart (between rows). For more information on production practices, visit the EDIS publication RFAC018, Opportunities for Small Farms: Peach and Nectarine Production Review (https://edis.ifas.ufl.edu/mg374).
Table 1. Perennial planting stock, growing media/location, and associated waiting period between establishment and time required before selling as USDA-certified organic.
Chill Requirements
Peach is a temperate-zone, deciduous fruit tree that requires cold temperatures (chill hours) during dormancy for optimum growth and fruit set. The minimum chill hours required for optimal fruit set are cultivar-specific and known as the chill unit requirement. Before breeding efforts from researchers at the University of Florida in the 1950s, most peach cultivars required approximately 650+ chill units, but now growers have the option for low-chill cultivars with requirements as low as 100 chill units (which permits peach production in central Florida). Chill units can be calculated by several models, but the model that defines a chill unit as 1 hour between 32°F to 45°F (0°C to 7°C) is appropriate for low-chill cultivars (Richardson et al. 1974; Sharpe et al. 1990). Florida growers can determine local chill unit accumulation data from the Florida Automated Weather Network (http://fawn.ifas.ufl.edu/) or the AgroClimate website (http://agroclimate.org/) and should select a cultivar suitable for their location. Peach trees will survive if the chill unit requirements are not met, but the tree may not bear fruit, may have an uneven bloom, and may have fruit with growth defects such as a bulging suture. For more information on cultivars, the chilling requirements, chilling unit map, marketing, labor, and planting guidelines, visit the EDIS publication RFAC018, Alternative Opportunities for Small Farms: Peach and Nectarine Production Review (https://edis.ifas.ufl.edu/ac018).
Beyond meeting the winter chill requirements, early ripening is also a critical trait for Florida production. The number of days from flowering to fruit set, or the fruit development period, is shorter for low-chill cultivars such as ‘Flordadawn’ that produces fruit in as little as 60 days after flowering. The most popular cultivars currently in production, ‘UFBest’, ‘TropicBeauty’, ‘UFSun’, and ‘UFOne’, have respective chill unit requirements of 100, 150, 100, and 150 hours and fruit respective development periods of 85, 89, 90, and 95 days. The UF/IFAS peach breeding program in the Horticultural Sciences Department is actively developing improved peach cultivars for Florida. For more information on available cultivars, visit the EDIS publication Cir1159, Florida Peach and Nectarine Varieties (https://edis.ifas.ufl.edu/mg374). Breeding within organic systems may produce cultivars with traits better suited for organic production, but the most important trait for all systems is chill hours.
Establishment
Site identification and preparation are also important to maximize tree and fruit growth. UF/IFAS Extension encourages all growers to submit a soil sample to a licensed soil laboratory prior to initially planting and yearly thereafter. Visit the Extension Soil Testing Laboratory's (ESTL) website (http://soilslab.ifas.ufl.edu/ESTL Home.asp) or check with your county Extension office for more information about how to collect and submit a soil, manure, or compost sample, and to obtain additional guidance about fertility management. Optimum soil nutrient levels for conventional and organic peach trees grown in Florida are still being developed.
Although peach leaves only need 50% of the intensity of full sun for maximum photosynthesis, planting in full sun is still recommended to account for cloud cover and to reduce leaf wetness. Plant trees in well-drained soils because most rootstocks are not tolerant to flooding and a higher density of trees can be planted on lighter soils. If possible, plant at higher elevations because flowers and growing fruits can be damaged by early-season freezes when colder air settles at lower elevations. Even at higher elevations, overhead irrigation that waters the entire tree during early-season freezes may be needed to avoid crop loss. For more information about specific weather conditions that will determine whether irrigation will be helpful or harmful to young fruit during cold weather, visit EDIS publication ABE 372, Using Psychrometric Chart for Frost Protection (https://edis.ifas.ufl.edu/ae406).
Trees can be transplanted at any time of the year. Transplants grown in pots are best planted after the last freeze in the spring, and bare-root transplants are best planted in the fall or winter. Trees should be placed in the ground, without stakes, such that the ground soil line is even or slightly above the media in the transplant pot or former soil line for bare-root trees. Holes should be twice as wide as the pot and can be amended with finished compost that is mixed with native soil prior to planting. Peach trees have been grown in large pots (such as a 5 gallon [640 fl oz] or larger pot) for experiments and where in-ground planting is not possible, but recommendations and production estimates for pot production have not been determined.
Sanitation Training, Pruning, and Thinning
Growers should clean tools to remove surface dirt and plant residues, and then sanitize with approved products before tree training and pruning to reduce the risk of spreading pathogens. Although rinsing with a sanitizer is not required for tools used exclusively in certified systems, growers opting to use one are required to follow dilution instructions on the product label. A chlorine-based sanitizer is the most commonly used sanitizer for nonfood contact surfaces, such as hand tools and trimming equipment. Some bleach products have surfactants or fragrances that are not allowed in organic production. See the NOP Handbook Section A. Standards 5026 and e-CFR § 205.601 for more information on approved sanitizers. More information on postharvest fruit sanitation is provided in the Harvest and Storage section below.
Most peach trees in the Southeast are trained so the overall shape resembles an open vase with the trunk and limbs resembling the stem and cup of a standard wine glass, respectively. Training involves cutting limbs on young trees to promote the development of three to six strong scaffold branches that are above and close to the rootstock/scion junction (2 ft or 61 cm above the ground). Branches extending from the rootstock itself should be removed and can easily be identified by their red leaves as opposed to the green leaves of the scion’s branches. Secondary and tertiary branches will grow off the scaffold branch and new flowers/fruit will develop on wood that grew in the previous year. After one to two years of pruning to develop the overall shape, yearly pruning is required.
In contrast to citrus, peach trees grown in Florida require two pruning events, one in the summer and one in the winter. Summer pruning occurs after harvest (postharvest pruning) in May and no later than June, and the main purpose is to maintain a reasonable height for the next harvest season with sufficient width to allow machinery to pass without damaging limbs. For more information about summer pruning, including pictures, visit EDIS publication HS1377, Summer Pruning in Low-Chill Peaches Grown in Florida (https://edis.ifas.ufl.edu/hs1377). Winter pruning occurs after the trees have entered dormancy (leaves have fallen) and occurs around December. The purpose of winter pruning is to remove dead wood, limbs growing straight up from the center of the tree (also called water sprouts), and other twigs that may bear leaves that impede air flow or damage growing fruit. For more information on pruning, including pictures, visit EDIS publication HS1111, Training and Pruning Florida Peaches, Nectarines, and Plums (https://edis.ifas.ufl.edu/hs365). After the last chance of a freeze, but before the peach fruit begin to rapidly increase in size, flowers and fruitlets should be removed by hand or with machinery (also called thinning) to a density of one fruit for every 6–8 inches (15–20 cm). To promote maximum fruit size, fruitlets and flowers should be thinned when the fruitlets are no longer than 1 inch (25 mm) from stem to tip and their stones are still soft (Figure 3). However, thinning later, after the stones harden and the fruit are longer than 1 inch, will not increase final fruit size. For more information about fruit thinning, including pictures, visit EDIS publication HS1324, Thinning Florida Peaches for Larger Fruit (https://edis.ifas.ufl.edu/hs1324).

Credit: David Campbell, UF/IFAS
Orchard Floor and Weed Management
Commercial orchards are planted in parallel rows, and the ground of the orchard can be divided into two sections, the area directly under the canopy (row middle) and between the trees (row alley). Row alleys typically consist of existing vegetation that is regularly mowed, while row middles are kept vegetation-free. Incorporation of cover crops in the row middle or row alley could improve soil health, but more information is needed regarding species selection and management to increase nutrient availability, reduce competition, and increase the number of beneficial insects while maintaining insect pests below acceptable levels. Maintaining vegetation, different tillage practices, and growing cover crops in the row middle and/or row alley have had positive (Reeve et al. 2013), negative (Antonelli et al. 1998), and insignificant (Belding et al. 2004) effects on peach trunk growth. Reducing peach tree competition for resources (water and fertility) with other plant species in the row middle when trees are first established is important for long-term orchard productivity. Row middle practices will need to be adjusted based on tree size and farm equipment. Before a large canopy develops, tillage is possible around young trees but may damage peach tree roots. When an orchard is initially established, practices that utilize heat (flaming or steam) can be effective methods to terminate emerging weeds in alleys, but growers need to be mindful to not damage young trees (Abulridha et al. 2019; Fennimore et al. 2017). A plant-based mulch (i.e., straw, pine bark) that is 3–4 inches (7–10 cm) thick can be used to reduce weed growth, but it will degrade, may affect nutrient availability, and will need to be replaced yearly. A manufactured mulch (i.e., landscape fabric or plastic) will persist longer than a plant-based mulch but may affect water penetration and other abiotic conditions, such as soil and canopy temperature. After attempting preventative weed management practices, organic compliant chemical weed management options are available with variable efficacy depending on species, age of weed, and rate of application. OMRI-certified inputs can be found at www.omri.org, but all growers need to consult with their certification agency before application.
Irrigation and Fertilization
Peach trees require a variable amount of water throughout the season. During dormancy, irrigation will likely not be needed, but it will be needed during flowering and fruit growth. Tree growth, soil, and meteorological conditions will determine how much irrigation is required. For more information, review HS1316, Irrigation Practices for Peaches in Florida, at https://edis.ifas.ufl.edu/hs1316. Peach trees require additional fertilization for optimum growth and productivity (Table 2), and a summary of fertility sources allowed in organic systems can be found in HS720, Introduction to Organic Crop Production (https://edis.ifas.ufl.edu/cv118).
Peach tree nutrient status can be determined by collecting leaf plus petiole tissues and submitting them to a laboratory for analysis. Samples can be collected at any time during the growing season to confirm a suspected deficiency, but growers wishing to validate their nutrient management program and plan for spring fertility applications should collect leaf samples after harvest (typically June) to correct any deficiencies (Table 3) while the trees are still growing. Soil tests are typically conducted after harvest and fertilizer applications adjusted to maintain proper fertility levels for the remaining and next year. Often, established organic systems require less nitrogen-based inputs than conventional systems to achieve similar yields due to increased nutrient-cycling efficiency (Halberg 1995). However, in newly established orchards, additional nitrogen (N) may be required to offset the decreased nutrient-cycling efficiency. Synchronizing N availability with crop demand is necessary, and growers will need to consider the applied product, application timing, temperature, and soil moisture in relation to N availability. Incubation studies that describe N release and plant availability for multiple organic-approved inputs have been conducted at soil temperatures similar to Florida summer (86°F or 30°C; Cassity-Duffey et al. 2020) and winter (73°F or 22.8°C; Lazicki et al. 2020), but additional studies are needed for locally available products. General observations from these studies show that most of the N is released from these products within 20 days and the N availability as a percentage of total N in the product over 100 days depended on material type: composted plant materials (~2%–4%), composted manure (~15%–30%), poultry litter (~20%–50%), and commercially blended fertilizers (~20%–80%). In a Florida study, researchers noted that compost with an 85% fertilizer efficiency, 2% N content, and a carbon:nitrogen ratio ≤ 25:1, applied at approximately 1.5 tons/acre, may satisfy the N requirement of a mature peach tree if applied in three applications (Litvany and Ozores-Hampton 2002). UF/IFAS N recommendations for conventional peach production are the only recommendations available at this time, and you are encouraged (and may be obligated, depending on your location in the state) to follow these recommendations. A recent fertigation study in Florida showed that excess applied nitrogen that does not leach through the soil is stored in the peach wood, including those branches that are removed in summer/postharvest pruning; therefore, the nitrogen is wasted because pruned branches are typically removed from the field (Rubio Ames et al. 2020). Notice nitrogen demand increases each year up to the fourth year (Table 2).
Table 2. Annual fertilizer recommendations for peach orchards in Florida.
Table 3. Peach leaf plus petiole deficiency and sufficiency ranges for selected nutrients.
Integrated Pest and Pathogen Management
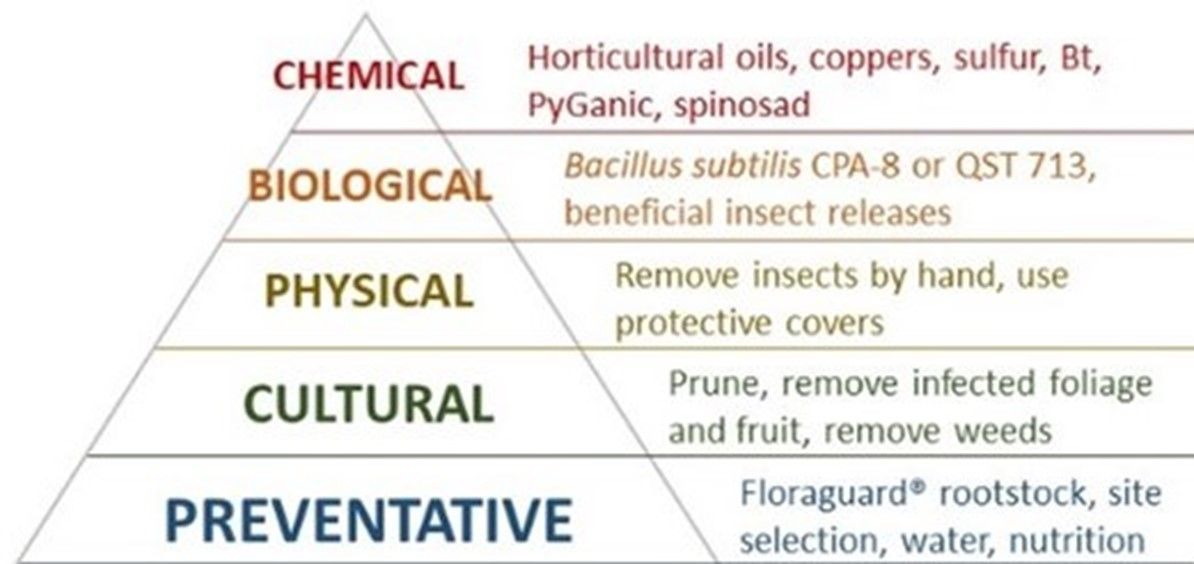
Insect and pathogen (Figure 4) management is one of the most challenging aspects of successful organic production. Growers employing the most effective organic compliant practices have claimed that over 50% of their crop had been lost under high temperature and humidity conditions that promote increased insect pest and pathogen activity. In decreasing order as represented from the bottom of the triangle to the top, organic integrated pest management relies on prevention, cultural management, physical management, biological control, and chemical applications as a last resort (Figure 5).

Credit: David Campbell, UF/IFAS
Credit: undefined
Cultural management, especially sanitation, is critical for insect and disease management. Diseased limbs and wounded/diseased fruit should be removed from the field to reduce fungal spore production and suitable habitats for insect pests. Producers may compost trimmings of diseased plant material according to the federal regulation (7 CFR § 205.203): “Composted plant and animal materials produced through a process that: i) Established an initial C:N ratio of between 25:1 and 40:1; and ii) Maintained a temperature between 131°F (55°C) and 170°F (76.7°C) for 3 days using an in-vessel or static aerated pile system; or iii) Maintained a temperature between 131°F and 170°F for 15 days using a windrow composting system, during which, the materials must be turned a minimum of five times.” However, some causative organisms will survive, despite using the best available methods for composting. The NOP guidelines are appropriate for management of human disease-causing pathogens. Although specific types of plant pathogens will die at 122°F (50°C) (nematodes, oocytes, and water molds), other plant pathogens only die at 180°F (82.2°C) (Agrios 2005).
Growers are encouraged to remove alternative vegetative hosts, such as wild plum [Prunus americana (Marsh.)] from within 600 ft (183 meters) to reduce brown rot (Monilinia fructicola) inoculum and broad leaf weeds to reduce the number of stink bugs (Order: Hemipteran) (Blaauw et al. 2022). Additional cultural management activities include using drip or microsprinkler irrigation at the base of the tree to avoid wetting the leaves, which may increase fungal spore growth and distribution, and regularly sanitizing all pruning tools (eOrganic 2020).
Producers with small orchards may remove insect pests using a vacuum or by hand (remove the insect pests and place them in a bucket of soapy water). However, this procedure is labor-intensive and impractical on a large scale. Other physical management options include spraying horticultural oils (Figure 5, Table 4) to provide a physical barrier on the fruit, leaves, limbs, and trunk. Recently, researchers found that manually bagging individual peach fruit with paper bags impregnated with wax reduced fruit fly oviposition by 82% and scab-like lesions (small black circular dots) by 84% (Campbell et al. 2021). Bags also potentially delayed maturity, but the practice of installing and removing bags is labor-intensive and highly dependent on labor and whether there is a market for this fruit.
Baited traps can be used to attract known pests, but it is unknown whether that technique is economically feasible to reduce fruit insect injury. Biological control agents, such as QST-713 or CPA-8 strains of Bacillus subtilis have been shown to produce an antifungal lipoprotein that suppresses brown rot, can be sprayed on leaves throughout the season, and possibly can be combined with foliar micronutrient applications (if allowed according to the label and the grower’s certification agency). Biological agents compete with microbial and fungal pathogens for food and resources on the leaves or exude chemicals that slow or stop pathogen growth. The applications of beneficial nematodes, Steinernema carpocapsae and Heterorhabditis bacteriophora, have been used successfully against peachtree borer (Synanthedon exitiosa) larvae when applied directly to cracks in the bark or to frass on the plant.
Action thresholds for chemical control of pests are site-specific, and although it is the last resort, growers regularly use organic compliant chemicals to control pests and pathogens. The Southeastern Peach, Nectarine, and Plum Management and Culture Guide (Blaauw et al. 2022) lists organic compliant chemical controls (Table 4), but all growers need to consult with their certification agency before application. In addition to these chemicals, growers in central Florida have been observed to rotate the QST 713 strain of Bacillus subtilis, pyrethrins, and a combination of rosemary oil/geraniol/peppermint oil after action thresholds have been reached. The effectiveness of many plant-based essential oils have been reported as having broad-spectrum activity against many plant pathogens, including fungi, bacteria and even soft-bodied arthropod pests (Calo et al. 2015; Nazzaro et al. 2017). Research on the effectiveness of eight essential oils to reduce Colletotrichum, Botryosphaeria, Fusarium, and Phytophthora activity on tropical fruits was promising (Sarkhosh et al. 2017; Sarkhosh et al. 2018a; Sarkhosh et al. 2018b). In 2021, a research team led by Sarkhosh tested Thymeguard, an OMRI-listed formulation of 23% thyme oil, on four strains of peach brown rot (Monilinia fructicola) grown on amended media under lab conditions. Preliminary results included a significant reduction in spore germination of all four strains on the amended media. In February 2022, the research team started an experiment applying Thymeguard every 10–14 days on organically grown peach trees to evaluate the efficacy of the product not only on brown rot but also on other peach tree diseases. Preliminary results for the field experiment will be concluded in May 2022, and additional testing will be conducted before official guidance is published.
Results from competitive grants that were awarded to other peach-producing regions (Pennsylvania and California) have provided positive experimental findings for pest and disease injury prevention (OFRF 1994; SARE 2013). While the research was completed on USDA-certified organic farms, the outcomes may differ in Florida. In Pennsylvania, researchers report that perennials grown in hedgerows near peach orchards in Pennsylvania harbor beneficial insects, such as a syrphid fly. They report that ladybug beetles control green peach aphids, but syrphid fly larvae were not observed in aphid colonies. Praying mantid adults and eggs were found in peach trees, coinciding with the increase in stink bug populations. Kaolin clay was applied to prevent plum curculio injury in this study, but the researchers did not measure efficacy (SARE 2013). In other studies, kaolin clay combined with selected plant extracts (Gӧkçe et al. 2013) increased insect pest aversion and effectively reduced oviposition in cotton (Showler 2002). Also, kaolin clay holds potential for controlling fruit flies, Anastrepha spp., in Florida.
In California, researchers reported that a mixture of Algrow kelp (3 lb or 1.36 kg), basalt rock dust milk (80 gal or 363.7 liters), and pink mucoid yeast (10 gal or 45.5 liters) sprayed on peach trees reduced brown rot. The researchers reported that 94% of the fruit did not have brown rot infection at harvest as compared to 58% for the untreated control. The Algrow kelp was a commercially available, cold-pressed, dry kelp product. The basalt rock dust milk was basalt rock dust mixed with 1 gal (153.7 fl oz; 4.6 liters) of a commercially available liquid kelp. The pink mucoid yeast was a cultured product with yeast that was scraped from peach leaves in the same orchard. Less effective treatments included hydrogen peroxide, compost tea (various), axomite clay and 1 gal (4.6 liters) liquid kelp, wine vinegar, raw apple cider vinegar, irrigation water with high algae content, and wettable sulfur (OFRF 1994). Producers using this approach in northern California have continued to update their materials to maintain compliance with the NOP, and an updated list of materials is available at eorganic.org in Table 3 in the Woodleaf Farm Organic Systems Description (Rosato et al. 2021). As always, please obtain approvals from your certification agency before making any changes to your organic system plan.
Harvest and Storage
The most practical ways to determine peach maturity are to observe the change in peel color (“ground color”) around the stem end from green to yellow/orange and to assess the firmness of the tip of the fruit (Brovelli et al. 1998). The red “blush” on the peel is formed in response to sunlight exposure and is not related to maturity. Peach fruit begin to ripen at the tip or blossom end and firmness should be assessed as the ground color begins to change. By grasping the fruit with the stem between the index and middle fingers, growers can easily assess the firmness by slightly pressing on the tip of the fruit with the thumb. Fruit to be marketed as “tree ripe” can be picked as soon as the tip begins to soften. During early ripening, the depressed tip will immediately resume the original shape, but if pressed too hard or assessed later in the season, the peel may break and/or the tip will remain depressed and vulnerable to fungal infection.
Peaches are climacteric, which means that when picked at the full mature stage, they can continue to ripen after harvest (unlike nonclimacteric fruit like citrus that can only ripen while on the tree). If harvested fruit are not cooled immediately, the ripening process will occur rapidly. If the fruit will be packed the following day, they should be cooled within a couple of hours of harvest to 45°F to 50°F (7°C to 10°C) using forced-air cooling or hydrocooling. However, if packing will occur even later, the fruit should be cooled to near 32°F (0°C) for storage up to 1 to 3 months at that temperature with a relative humidity at 90% to 95%. Storage of peaches at 36°F to 45°F (2°C to 7°C) can favor development of a disorder called chilling injury or “internal breakdown,” which causes undesirable sensory perception characterized as lack of aroma, “woolly” or mealy, dry pulp texture, and reddish-brown flesh discoloration. Lower temperatures inhibit the development of chilling injury symptoms. Therefore, long-term storage should be below the 36°F to 45°F temperature range, and temporary storage should be at nonchilling temperatures above that range. The freezing point of peach fruit varies from 27°F to 30°F (-3°C to -1°C) depending on the concentration of sugars in the fruit—higher sugar content corresponds to lower freezing point (Gross et al. 2016).
Peach brown rot is a very common postharvest storage decay caused by the fungus Monilinia fructicola, which infects fruit in the orchard. In addition to the benefit of cooling in reducing postharvest decays, bagging young fruit in the field reduces pathogen infection so that the likelihood of fruit developing brown rot at harvest is reduced by 69% (Figure 6) (Campbell et al. 2021). Following bag removal at the time of harvest, after 7 days of storage, brown rot decreased by 32%. For more information about peach brown rot including pictures, visit the EDIS publication HS1357, Peach Brown Rot (https://edis.ifas.ufl.edu/hs1357).

Credit: David Campbell, UF/IFAS
The populations of microbes that cause foodborne human illnesses and postharvest fruit decays can also be reduced with organic compliant sanitizers. Approved sanitizers include ozone (requires special equipment), peroxyacetic acid, acetic acid, hydrogen peroxide, and low concentrations of chlorine (hypochlorite ion; bleach). Chlorine must be labelled specifically for sanitation (food grade) and can be purchased locally, but an extra rinse with potable water is required before shipping. Other products can be distinguished by an OMRI label (eOrganic 2020). Growers should be mindful of fruit and water temperatures during hydrocooling, which requires the use of sanitizer in the recirculated water to minimize cross-contamination of fruit. Fruit will absorb water (and sanitizer) during the cooling process if the fruit are immersed in water that is ≥10°F (-12°C) cooler than the fruit. If the sanitizer, such as chlorine, requires an additional rinse before sale, then the fruit that absorbed sanitizer would no longer be processed according to organic standards and, hence, cannot be labeled as such. Shower-type hydrocoolers largely avoid this immersion-absorption issue.
Check with your certifying agent before applying fruit coatings to ensure the product is allowed for use. Packaging materials, including bags, bins, and shipping containers that contact the fruit must be new, or if reused or shared with a noncertified segment of your operation, must be thoroughly cleaned (and documented as such) before using in your organic system. Organic peaches must be kept separate from conventional produce during harvest and collection operations as well as during cooling, packing, storage, transportation, and display shelves in stores to prevent contact with surfaces that may have residues of prohibited products. This is referred to as comingling.
For more information on postharvest handling of specialty crops, please review the UF/IFAS EDIS publication, HS1270, Postharvest Storage, Packing and Handling of Specialty Crops: A Guide for Florida Small Farm Producers at https://edis.ifas.ufl.edu/hs1270 or the University of California Postharvest Center’s Management for Organic Fruit and Vegetable Crops at http://postharvest.ucdavis.edu/Online_Extension_to_Educate_Small_Farms/Postharvest_Management_for_Organic_Fruit_and_Vegetable_Crops/.
Table 4. Pests and management options for organic peach production systems in Florida. This table is a summary of compliant approaches only, and it does not replace the product label. Verify compliance and receive approval for use from your certification agency before using any products in your transitioning or certified system. For the most current recommendations and label instructions, please visit the Southeastern Peach, Nectarine, and Plum Pest Management and Culture Guide. Please note this guide contains prohibited and approved products that have been tested and determined to be effective in the Southeast. See Pesticide experts include (1) Insecticides and Miticides: Oscar E. Liburd, UF/IFAS Entomology and Nematology Department; (2) Fungicides and Bactericides: Phil Harmon, UF/IFAS Plant Pathology Department; and (3) Organic Compliance: Danielle Treadwell, UF/IFAS Horticultural Sciences Department.
Other Relevant EDIS Publications
Note: Some of the UF/IFAS Extension EDIS publications listed below provide useful information to all producers but may mention conventional inputs that are prohibited in organic systems; please verify compliance before use with your certification agency.
- Cir1435, Calibration of Airblast Sprayers: http://edis.ifas.ufl.edu/ae238
- ENY683, Xylella Fastidiosa Diseases and Their Leafhopper Vectors: https://edis.ifas.ufl.edu/in174
- ENY691, Peachtree Borers in the Home and Commercial Peach Orchard: https://edis.ifas.ufl.edu/in489
- ENY822, Natural Enemies and Biological Control: https://edis.ifas.ufl.edu/in120
- FPS492, Prunus americana American Plum: https://edis.ifas.ufl.edu/fp492
- HS720, Introduction to Organic Crop Production: https://edis.ifas.ufl.edu/cv118
- HS1111, Organic Vegetable Gardening in Florida: https://edis.ifas.ufl.edu/hs1215
- HS1249, Peach Scab: https://edis.ifas.ufl.edu/hs1249
- HS1263, Peach Rust (Transchelia spp.): https://edis.ifas.ufl.edu/hs1263
- HS1265, Fungal Gummosis in Peach: https://edis.ifas.ufl.edu/hs1265
- SL443, Tools for Evaluating Soil Health: https://edis.ifas.ufl.edu/ss657
References
Abulridha, J. J., R. G. Kanisserty, C. E. McAvoy, and Y. G. Ampatzidis. 2019. “Evaluation of Steam Application for Weed Management in Citrus.” Applied Engineering in Agriculture 35 (5): 805–814. https://doi.org/10.13031/aea.13494
Agrios, G. N. 2005. “Chapter 9. Control of Plant Diseases.” In Plant Pathology (5th edition), pp. 293–353. Amsterdam: Elsevier. https://doi.org/10.1016/B978-0-08-047378-9.50015-4
Antonelli, M., A. Chiariotti, M. R. Tabilio, M. Ronco, and P. Di Prospero. 1998. “Effects of Different Management Techniques Utilized in an Organic Peach Orchard during the Training Phase.” Acta. Hort. 465:587–592. https://doi.org/10.17660/ActaHortic.1998.465.73
Belding, R. D., B. A. Majek, G. R. Lokaj, J. Hammerstedt, and A. O. Ayeni. 2004. “Orchard Floor Management Influence on Summer Annual Weeds and Young Peach Tree Performance.” Weed Technol. 8 (2): 215–222. https://doi.org/10.1614/WT02-180
Blaauw, B., P. Brannen, B. Bellinger, D. Lockwood, and D. Ritchie. 2022. “Southeastern Peach, Nectarine and Plum Pest Management and Culture Guide.” Bul. 1171. University of Georgia Cooperative Extension Service. http://extension.uga.edu/publications/detail.html?number=B1171
Brovelli, E. A., J. K. Brecht, W. B. Sherman, and C. A. Sims. 1998. “Potential Maturity Indices and Developmental Aspects of Melting-Flesh and Nonmelting-Flesh Peach Genotypes for the Fresh Market.” J. Amer. Soc. for Hort. Sci. 123 (3): 438–44. https://doi.org/10.21273/JASHS.123.3.438
Calo, J. R., P. G. Crandall, C. A. O'Bryan, and S. C. Ricke. 2015. “Essential Oils as Antimicrobials in Food Systems: A Review.” Food Control. 54:111–119. https://doi.org/10.1016/j.foodcont.2014.12.040
Campbell, D., A. Sarkhosh, J. Brecht, J. Gillett-Kaufman, O. Liburd, J. C. Melgar, and D. Treadwell. 2021. “Bagging Organic Peaches Reduces Physical Injuries and Storage Decay with Minimal Effects on Fruit Quality.” HortScience 56 (1): 52–58. https://doi.org/10.21273/HORTSCI15391-20
Cassity‐Duffey, K., M. Cabrera, J. Gaskin, D. Franklin, D. Kissel, and U. Saha. 2020. “Nitrogen Mineralization from Organic Materials and Fertilizers: Predicting N Release.” Soil Science Society of America Journal 84 (2): 522–533. https://doi.org/10.1002/saj2.20037
eOrganic. 2020. “Approved Chemicals for Use in Organic Postharvest Systems.” https://eorganic.org/node/2669
Ferguson, J., J. Chaparro, J. G. Williamson, R. Rouse, and R. Mizell. 2007. “Florida Subtropical Peaches: Production Practices.” EDIS 2007 (20). https://doi.org/10.32473/edis-hs348-2007
Gӧkçe, A., L. L. Stelinski, D. R. Nortman, W. W. Bryan, and M. E. Whalon. 2014. “Behavioral and Electroantennogram Responses of Plum Curculio, Conotrachelus nenuphar, to Selected Noxious Plant Extracts and Insecticides.” J. of Insect Sci. 14 (1): 90. https://doi.org/10.1093/jis/14.1.90
Gross, K.C., C.Y. Wang, and M.E. Saltveit (eds.). 2016. “The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Stocks.” United States Department of Agriculture, Agricultural Research Service. Agricultural Handbook 66. https://www.ars.usda.gov
Halberg, N., E. S. Kristensen, and I. S. Kristensen. 1995. “Nitrogen Turnover on Organic and Conventional Mixed Farms.” J. of Agr. and Environ. Ethics 8 (1): 30–51. https://doi.org/10.1007/BF02286400
Handoo, Z. A., A. P. Nyczepir, D. Esmenjaud, J. G. van der Beek, P. Castagnone-Sereno, L. K. Carta, A. M. Skantar, and J. A. Higgins. 2004. “Morphological, Molecular, and Differential Host Characterization of Meloidogyne floridensis n. Sp. (Nematoda: Meloidogynidae), a Root-Knot Nematode Parasitizing Peach in Florida.” J. of Nematol. 36 (1): 20–35.
Johnson, R. S. 2008. “Nutrient and Water Requirements of Peach Trees.” In The Peach: Botany, Production and Uses, edited by D. R. Layne and D. Bassi, 303–321. Cambridge, MA: CAB International. https://doi.org/10.1079/9781845933869.0303
Lazicki, P., D. Geisseler, and M. Lloyd. 2020. “Nitrogen Mineralization from Organic Amendments Is Variable but Predictable.” J. of Environ. Qual. 49 (2): 483–495. https://doi.org/10.1002/jeq2.20030
Litvaney, M., and M. Ozores-Hampton. 2002. “Compost Use in Commercial Citrus in Florida.” HortTechnology 12 (3): 332–335. https://doi.org/10.21273/HORTTECH.12.3.332
Mancero-Castillo, D., A. Sarkhosh, C. Ligon, M. Olmstead, and P. Harmon. 2018. “Peach Rust (Transchelia spp.).” EDIS 2018 (4). https://doi.org/10.32473/edis-hs1263-2018
Nazzaro, F., F. Fratianni, R. Coppola, and V. De Feo. 2017. “Essential Oils and Antifungal Activity.” Pharmaceuticals (Basel) 10 (4): 86. https://doi.org/10.3390/ph10040086
OFRF. 1994. “Peach Brown Rot Control.” Organic Farming Research Foundation (OFRF) Grant Report 92-26. 1–12. Available upon request from OFRF.
PennState Extension. 2022. “Tree Fruit Production Guide: Reentry and Perharvest Intervals.” AGRS-045. https://extension.psu.edu/reentry-and-preharvest-intervals-antibiotics-and-fungicides
Perez, A., and K. Plattner. 2013. “Fruit and Tree Nuts Outlook: Commodity Highlight. Organic Fruit and Berries.” USDA Economic Research Service. FTS-356SA. https://www.ers.usda.gov/webdocs/outlooks/37053/41059_fts-356sa.pdf?v=9293.4.
Phillips, D. A., P. J. Dittmar, P. Harmon, O. E. Liburd, D. D. Treadwell, and J. G. Williamson. 2021. “Organic Blueberry Production in Florida.” EDIS 2021 (1). https://doi.org/10.32473/edis-hs1400-2021
Reeve, J. R., C. M. Culumber, B. L. Black, A. Tebeau, C. V. Ransom, D. Alston, M. Rowley, and T. Lindstrom. 2017. “Establishing Peach Trees for Organic Production in Utah and the Intermountain West.” Sci. Hort. 214:242–51. https://doi.org/10.1016/j.scienta.2016.11.040
Rosato, C., H. Atthowe, and A. Stone. 2021. “Organic Farm System: Woodleaf Farm.” https://eorganic.org/node/14129
Rubio Ames, Z., J. K. Brecht, and M. A. Olmstead. 2020. “Nitrogen Fertilization Rates in a Subtropical Peach Orchard: Effects on Tree Vigor and Fruit Quality.” J. Sci. Food Agric. 100 (2): 527–539. https://doi.org/10.1002/jsfa.10031
SARE. 2013. “Evaluation of 12 Yellow Flesh Peach Cultivars for Organic Production in the Northeast: Final report for FNE11-730.” Sustaniable Agriculture Research and Education Projects. https://projects.sare.org/project-reports/fne11-730/
Sarkhosh, A., B. Schaffer, A. I. Vargas, A. J. Palmateer, P. Lopez, A. Soleymani, and M. Farzaneh. 2018a. “In Vitro Evaluation of Eight Plant Essential Oils for Controlling Colletotrichum, Botryosphaeria, Fusarium, and Phytophthora Fruit Rots of Avocado, Mango, and Papaya.” Plant Protection Sci. 54:153–162. https://doi.org/10.17221/49/2017-PPS
Sarkhosh, A., B. Schaffer, A. I. Vargas, A. J. Palmateer, P. Lopez, A. Soleymani, and M. Farzaneh. 2018b. “Antifungal Activity of Five Plant-Extracted Essential Oils against Anthracnose in Papaya Fruit.” Biological Agric. and Hortic. 34 (1): 18–26. https://doi.org/10.1080/01448765.2017.1358667
Sarkhosh, A., A. I. Vargas, B. Schaffer, A. J. Palmateer, P. Lopez, A. Soleymani, and M. Farzaneh. 2017. “Postharvest Management of Anthracnose Affecting Stored Avocado (Persea americana Mill.) Fruit with Plant-Extracted Essential Oils.” Food Packaging and Shelf Life 12:16–22. https://doi.org/10.1016/j.fpsl.2017.02.001
Showler, A. T. 2002. “Effects of Kaolin-Based Particle Film Application on Boll Weevil (Coleoptera: Curculionidae) Injury to Cotton.” J. of Econ. Entomol. 95 (4): 754–762. https://doi.org/10.1603/0022-0493-95.4.754
Tucker, J. 2019. “USDA AMS Policy Memo to USDA-Accredited Certifying Agents. Certification of Organic Crop Container Systems.” 3 June 2019. https://www.ams.usda.gov/sites/default/files/media/2019-Certifiers-Container-Crops.pdf
USDA ERS. 2020. “Data by Commodity—Imports and Exports.” 21 Jan 2021. https://data.ers.usda.gov/reports.aspx?programArea=fruit&top=5&HardCopy=True&RowsPerPage=25&groupName=Noncitrus&commodityName=Peaches&ID=17851#P68de095f55b747c4b37ef7c871c0aeed_2_292
USDA NASS. 2020. “2017 Census of Agriculture. 2019 Organic Survey.” United States Department of Agriculture, National Agricultural Statistics Service. https://www.nass.usda.gov/Publications/AgCensus/2017/Online_Resources/Organics/index.php