Many tomato diseases occur in open-field and high-tunnel production systems; however, some diseases (e.g., leaf mold, gray mold) are more common in high-tunnel production due to the unique microenvironments observed in enclosed structures. Disease identification and monitoring are critical components of an integrated disease management program that utilizes resistant varieties and cultural practices (e.g., irrigation management and soil solarization or disinfestation). This article provides a concise overview of some influential high-tunnel tomato soilborne and foliar diseases and their cultural management techniques.
Foliar Fungal Diseases
Leaf Mold (Passalora fulva and Psudocercospora fuligena)
Symptoms and Signs: Leaf mold can be caused by two distinct fungi (P. fulva and P. fuligena) and is a common issue in high-tunnel and greenhouse tomato production systems. Symptoms can be observed as light-green or yellow spots on the upper leaf surface. The disease progresses showing fungal signs on the lower surface as a velvety, tan growth underneath the spots. The later stages of this disease causes blighting and premature leaf dropping that can lead to severe defoliation. Leaf mold development is favored by high relative humidity (>85%) (Thomma et al. 2005) and warm temperatures (~83°F).

Credit: Zach Eldred, UF/IFAS
Cultural Management: Crop residues, especially infected tissue, should be removed and destroyed whenever possible and especially after harvest. Be sure to sanitize the high tunnel between tomato production cycles, which can be achieved with a pressure washer or sterilizing products containing alcohol, sodium hypochlorite or ammonium (Smith et al. 2019). Pruning can increase ventilation and slow this disease. Commercial varieties with resistance to leaf mold, Passalora fulva, are available and act as an effective prevention method.
Botrytis Gray Mold (Botrytis cinerea)
Symptoms and Signs: Gray mold develops during moderate temperatures (65°F to 75°F) and high relative humidity (>80%). Symptoms can be observed on all aboveground plant parts. A sign of gray mold is the unique fuzzy appearance on infected tissue, which is typically first found on dead or dying flowers. Stem lesions may occur near or at the soil line and progress up the stem over time, often girdling the plant and leading to death. Infections are also common on leaflets as well as the flowers and fruits. Soft rot symptoms on fruits, typically observed as mushy and watery tissue, are common for this disease. Additionally, the immature fruit may exhibit necrotic or whitish halos called “ghost spots”; when fruit mature the halos turn from white to yellow.

Credit: Gerald Holmes, Strawberry Center, Cal Poly San Luis Obispo, Bugwood.org
Cultural Management: Currently, there are no resistant varieties to this pathogen. An integrated approach utilizing multiple techniques should be used to prevent and limit the spread of this pathogen. Effective controls include giving adequate spacing between plants, pruning to increase ventilation, and improving sanitation by removing sources of inoculum such as infected flowers and debris. Additionally, pruning to avoid petiole stubs and when conditions (low humidity and free moisture) favor healing has effectively reduced Botrytis stem canker infections (Decognet et al. 2010).
Powdery Mildew (Pseudoidium neolycopersici and Leveillula taurica)
Symptoms and Signs: Fungal signs (e.g., spores) are commonly found on the upper leaf surface, but this can vary between pathogens. Symptoms start as light-green to yellow blotches or spots that gradually change to brown. Eventually leaves will turn brown to dark brown and shrivel while remaining attached to the stem. This pathogen prefers moderate temperatures (50°F to 75°F) and relative humidity (<90%), and it requires little to no water on the leaf surface for development. High relative humidity (>95%) can limit this pathogen. The spread of this pathogen most commonly occurs by wind dispersal of the powdery spores on the leaf surface.

Credit: Zach Eldred, UF/IFAS
Cultural Management: Plant resistant varieties whenever possible. Avoid pathogen spread by limiting weeds or volunteer plants that may harbor the pathogen. This includes sanitizing the high tunnel and removing diseased plant material or tilling in plant residues between tomato production cycles. Additionally, the use of disease-resistant varieties/cultivars when paired with integrated management techniques (prevention, chemical, etc.) can improve management (see varieties below).
Early Blight (Alternaria linariae)
Symptoms and Signs: The disease symptoms can be observed on the leaves, stems, and fruit. Pronounced leaf symptoms of the lower canopy are most common. Foliar symptoms are brownish to black lesions, which enlarge presenting concentric circles. Lesions on the stems are dark and sunken, also enlarging concentrically. Symptoms observed on the fruit are dark lesions that enlarge concentrically and can mature into a black velvety mass. This fungus is observed to spread rapidly during warm (60°F to 90°F) and wet (≥90% relative humidity and/or free moisture) conditions. Early blight is one of the most common tomato diseases, but previous studies have indicated that high tunnels may reduce disease severity compared to open-field production systems (Frey et al. 2020; Rogers and Wszelaki 2012).

Credit: Zach Eldred, UF/IFAS
Cultural Management: Use available varietal resistance to reduce leaf and stem infections. If possible, prevent lower leaves from contacting bare soil by staking or trellising, pruning, and barriers such as plastic or organic mulches. Clean and sanitize all pruning tools regularly to avoid mechanical spread. Remove and destroy any remaining infected plant material and weeds between crop cycles. The addition of UV-absorbing vinyl films may also help slow disease spread (Fourtouni et al. 1998).
Soilborne Fungal Diseases
Fusarium Wilt (Fusarium oxysporum f. sp. lycopersici)
Symptoms and Signs: This pathogen enters susceptible plants through wounds and natural openings in the roots. Symptoms can include seedling stunting, a pronounced yellowing of leaves, and eventual wilting of the plant. Symptoms of yellowing and wilting often begin on lower leaves and progress upwards over time, and they may even only affect a portion of the plant or leaf. Symptom development is enhanced during warm growth periods or when the plant begins to bear fruit. In addition, plants will develop a distinct yellow or brown discoloration of the vascular tissues, extending from the crown into upper symptomatic tissues. This disease will eventually lead to the plant death.

Credit: Mathews Paret, U-Scout
Cultural Management: Resistant varieties are commercially available and are often labeled with VFN, which stands for Verticillium, Fusarium, and nematode resistance. However, the effectiveness of resistance can vary depending on the fungal and nematode population in your field. It is recommended that producers seek the guidance of a specialist to identify the population present in your field when selecting a resistant cultivar. Grafting with resistant rootstocks and planting disease-free transplants are effective methods for managing Fusarium wilt. Take precautions not to move contaminated soil from infested high tunnels by cleaning tools and clothes before working in other tomato production areas. Use new wooden stakes or sterilize before reusing old ones. If possible, do not use ammoniacal nitrogen. Adjusting pH to 7.0 has been shown to reduce pathogen incidence. Rotate with a nonsusceptible plant, such as leguminous crops. Techniques such as anaerobic soil disinfestation, soil solarization, fumigation, steaming, and rotation of high tunnel location can all be effective at mitigating disease.
Fusarium Crown and Root Rot (Fusarium oxysporum f. sp. radices-lycopersici)
Symptoms and Signs: This pathogen, which is very similar to the Fusarium wilt pathogen, enters susceptible plants through the natural openings caused by the formation of new roots. This disease is concentrated in the roots and crown region near the soil line. Foliar symptoms include a rapid wilting that may include some yellowing foliar tissues, but not to the same extent as seen with Fusarium wilt, and frequently includes wilting of green tissues. Root and crown symptoms consist of a yellow to brown discoloration in the vascular tissue that do not progress more than 8 to 12 in. above the soil line (Zhang et al. 2021). In addition, plants develop a general root rot of tap and lateral roots as well as brown stem lesions at the soil line that can girdle the crown, which often leads to the extensive production of adventitious roots at the crown. Disease progression eventually leads to plant death. Unlike Fusarium wilt, this disease is favored by cooler temperatures (50°F to 68°F) (Zhang et al. 2021).
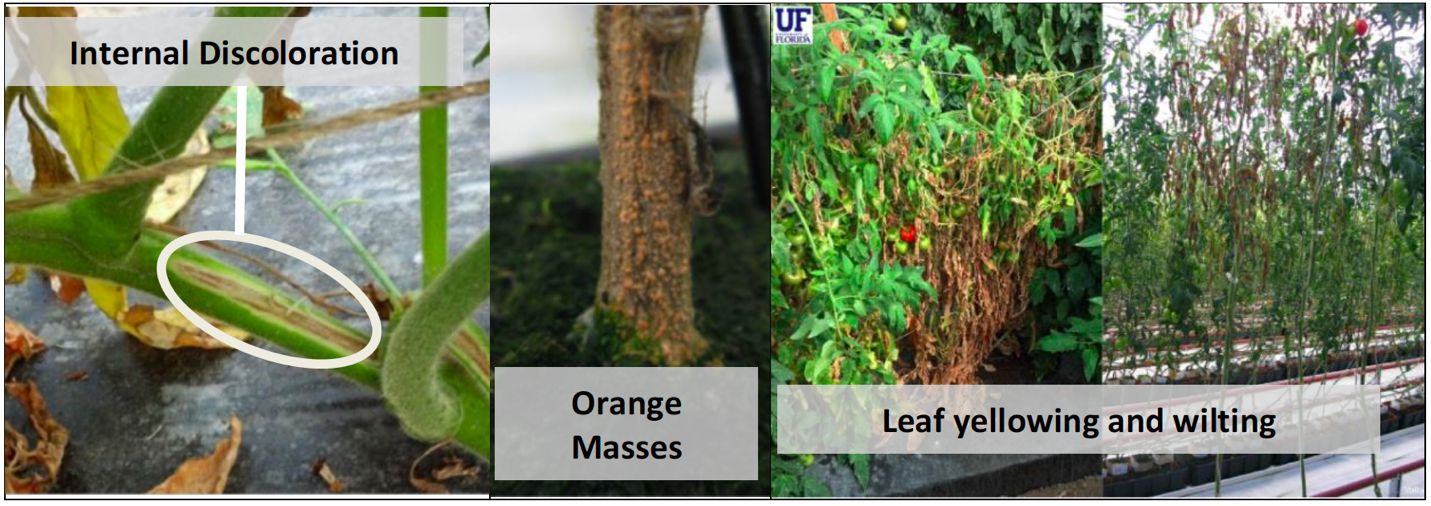
Credit: Mathews Paret, U-Scout
Cultural Management: All attempts should be made to mitigate this disease through an integrated management approach, including using disease-free transplants, utilizing either resistant varieties or grafting with a resistant rootstock, rotating to a nonsusceptible host (leguminous crops), and employing preplant soil disinfestation/sterilization. Removal of plant debris during and at the end of the season is critical as well as avoiding transport of soil to other locations. Sanitation of tools, equipment and clothes is recommended when dealing with a disease outbreak.
White Mold or Sclerotinia Blight (Sclerotinia sclerotiorum, S. minor and S. trifoliorum)
Symptoms and Signs: This disease typically appears at flowering but can be observed earlier in high tunnels. Symptoms will start out as water-soaked areas and expand into large cankers on the stems and branches. White mycelia can grow out of the infected area with the vascular tissue having irregular-shaped, black sclerotia. Shortly after symptom development, the infection will quickly kill the stem and then the whole plant. This pathogen can infect the stems, flowers, and fruit.

Credit: Zach Eldred, UF/IFAS
Cultural Management: Deep plowing, crop rotation, anaerobic soil disinfestation, soil fumigation, and various soil amendments may be useful in managing this pathogen. Immediate plant removal is necessary to prevent further spread of this pathogen. An integrated approach with several practices will be needed to manage this disease as infections can occur from local (within the tunnel) or distant (outside the tunnel) inoculum sources.
Southern Blight (Athelia rolfsii)
Symptoms and Signs: This pathogen is not often detected until moderate to severe symptoms of wilting begin to occur. However, initial symptoms of this disease are generally water-soaked lesions at the crown of the plant, on or near the soil line. Signs of this pathogen are seen as a growth of white mycelia, which form a mat around the infected plant parts. This pathogen will produce many tan, round, mustard-seed-sized sclerotia where there is an infection and mycelial growth. The fruits that come in contact with bare soil may also become infected and produce hyphal mats and sclerotia, leading to fruit rot.

Credit: Mathews Paret, U-Scout
Cultural Management: Effective management of this pathogen begins with avoiding planting in fields with a history of the disease. Pathogen exclusion is critical to preventing the introduction of this pathogen in the field. Utilizing clean media and ensuring that transplants are disease-free can help reduce the probability of introducing the pathogen into the field. Immediately remove infected plants and surrounding soil being careful to limit spread to adjacent plants. Soil treatments such as soil solarization and disinfestation can reduce the presence of the pathogen. Deep plowing and burying of planting material has also been shown to be effective at managing this disease.
Viral Diseases
Tomato Yellow Leaf Curl Virus (TYLCV)
Symptoms and Signs: This pathogen is transmitted by the adult silverleaf whitefly. The whitefly can transmit TYLCV for 10 to 12 days, and it can acquire the virus multiple times in a season. Symptoms are typically seen first on the newest leaves as marginal yellowing, or chlorosis, and leaf cupping. Additional symptoms may be seen as mottling, reduced leaf size, stunting, and fruit and leaf drop depending upon the age of the plant. One or more of the symptoms may be observed at one time and become more pronounced with plant age. Symptoms of this disease are obvious but not unique, so laboratory diagnosis is recommended.

Credit: Juan Diaz-Perez, UGA, and Nicholas Dufault, UF/IFAS
Cultural Management: Select varieties with resistance traits for TYLCV and use virus- and whitefly-free transplants. Rogue any diseased plants. Effective whitefly management is critical to reduce seasonal spread of the virus. Use a fallow period between crops and/or appropriate crop rotation practices to reduce whitefly vector buildup (Smith et al. 2018). After the growing season, immediately remove or destroy all tomato plants. Practice good weed management in and around the structure to the largest extent that is feasible. Even if TYLCV-tolerant varieties are utilized, whitefly populations must still be managed, because high whitefly populations can lead to uneven fruit ripening. Tolerant varieties will still exhibit mild viral symptoms upon infection and can serve as a source of the virus.
Tomato Chlorosis Virus (ToCV)
Symptoms: This disease occurs sporadically throughout Florida. The virus is most effectively transmitted by the silverleaf whitefly for about 1 to 10 days after acquiring the virus (Simone et al. 1996). Symptoms are typically seen on the bottom half of the plant, with the leaves becoming yellow between the veins. ToCV symptoms are often mistaken for magnesium deficiency or root-knot nematode damage and are associated with other types of tomato “yellows” diseases. The progression of the disease begins in the lower leaves and eventually moves to the new growth, with the leaves eventually becoming brittle or thick with necrotic flecks (Simone et al. 1996). Additionally, fruit development may be stunted due to the reduced photosynthetic capacity caused by this virus.

Credit: Zach Eldred and Nicholas Dufault, UF/IFAS
Cultural Management: Use certified pathogen-free seed if available and virus- and whitefly-free transplants. Also, remove transplant seedlings that have issues present and send for testing when possible. Use a fallow period between crops and/or appropriate crop rotation practices to reduce whitefly vector populations (Smith et al. 2018). Rogue out any early symptomatic plants to limit viral spread. After the growing season, immediately remove or destroy all tomato plants. Eliminate solanaceous weeds in and around the structure to the largest feasible extent.
Tomato Spotted Wilt Virus (TSWV), Groundnut Ring Spot Virus (GRSV) and Tomato Chlorotic Spot Virus (TCSV)
Symptoms and Signs: These pathogens belong to the viral family Tospoviridae, which is transmitted by thrips. Thrips can only acquire the virus during the larval stage but may transmit the virus during either the larval or adult stage. Symptoms begin on the leaves as light-brown flecks, eventually becoming brown and resulting in death. Symptoms caused by this pathogen are often mistaken for a wilt pathogen because of its droopy leaves and stunting. The symptoms that occur on the fruit are the most striking with this disease and can be seen as yellow or brown concentric rings that alternate with the color on green fruit, while the rings on the ripe red fruit are conspicuously brown.

Credit: Mathews Paret, U-Scout
Cultural Management: Use resistant varieties whenever possible. Use transplants that are free of thrips and other issues. Consider rogueing young seedlings that show symptoms. Manage weeds and practice sanitation measures to manage thrip populations. Other practices, such as planting noncrop plants to indicate when thrips are present, also known as indicator plants, are useful in limiting disease spread.
Tobacco Mosaic Virus (TMV) and Tomato Mosaic Virus (ToMV)
Symptoms and Signs: This pathogen is a mechanically transmitted, seed-borne virus that can result in severe losses. Symptoms of TMV often vary between plant cultivars, age of the plant, environmental conditions, and strain of the virus. However, many common symptoms include light-yellow-green to dark-green mosaic patterns on the leaves. Malformation or distortion of the leaves may occur, often referred to as “leaf strapping” or a “shoestring-like” appearance. Infection may also cause mosaic or necrotic patterns on the fruit, which may lead to premature ripening and internal browning. Advanced symptoms of this pathogen may cause severe leaf yellowing with green veins.

Credit: Mathews Paret, U-Scout
Cultural Management: Use resistant varieties whenever possible. Refrain from handling plants when using tobacco products, and wash all tools with either soap, 0.06% sodium hypochlorite (bleach) solution, or 2% Virikon S (Li et al. 2015) when pruning to prevent viral spread. Take care to avoid spreading the virus from infected plants to healthy plants by workers and equipment. If possible, dig up and remove infected plants from the field to reduce inoculum. Ensure to always use disease-free or treated seeds. Rotate with nonhost crops whenever possible.
Nematodes
Root-Knot Nematode (RKN—Meloidogyne spp.)
Symptoms and Signs: The root-knot nematode, a microscopic worm, is a plant-parasitic nematode that commonly damages tomatoes in Florida (Crow 2020). The symptoms from this parasite are due to the disruption in normal function of the root system. Initial symptoms are often associated with nutrient deficiency, such as leaf yellowing (chlorosis), stunting, and a general decline in plant vigor. Wilting occurs in severe cases but may recover when irrigated. Symptomatic plants are generally distributed in patches and are characterized by the unique knots or galls present on the root system. This parasite is often a severe problem in sandy soils, and high tunnels may create a unique environment for greater RKN infestations than open-field production systems (Frey et al. 2020).
Cultural Management: Use resistant varieties wherever possible. Rotate between nonhost crops, such as cover crops that suppress RKN (sunn hemp, marigold, cowpea, etc.) (Gill and McSorley 2017). Destroy all crop residues quickly after harvest. Other practices, such as soil solarization, may be used depending on soil type and resource availability.
Chemical and Biological Controls
Many of the diseases mentioned can be managed using chemical means alone or as part of an integrated program. Management using chemical and biological products requires (1) an accurate diagnosis of the disease(s), (2) a thorough understanding of the product’s efficacy and the labeled usage, (3) an understanding of the product’s shelf life/expiration date, and (4) storing the product in the proper conditions and facilities. For a list of products available, see chapters 18 and 19 of the 2022–2023 Vegetable Production Handbook of Florida, “Tomato Production” (https://edis.ifas.ufl.edu/publication/CV137) and “Biopesticides and Alternative Disease and Pest Management Products” (https://edis.ifas.ufl.edu/publication/CV295).
Resistance Varieties and Cultivars
Host resistance is one of the most effective tools for preventing and reducing the impact of plant diseases. However, the effectiveness of a host resistance trait can vary between varieties/cultivars, with the pathogen populations that are present and in relation to various environmental conditions (e.g., extreme weather). For example, the Fusarium wilt pathogen is composed of multiple “races” that require specific host resistant traits. Without these specific traits, tomato plants are susceptible to the pathogen, which is why contacting a specialist was recommended above.
The commercial availability of resistant tomato varieties continues to grow each year. Often these varieties contain traits that can prevent multiple diseases. Information about host resistance can be found on the seed packaging or in technical/specification sheets. However, if there is a question about a variety’s resistant traits, it is advisable to contact the seed producing company with the information provided on the label. A summary of tomato cultivars and their respective resistance traits can be found in the Vegetable Production Handbook of Florida chapter on tomatoes (https://edis.ifas.ufl.edu/publication/CV137).
References
Crow, W. T. 2020. “Nematode Management in the Vegetable Garden.” EDIS 1997 (2). https://edis.ifas.ufl.edu/publication/NG005
Decognet, V., F. Ravetti, C. Martin, and P. C. Nicot. 2010. “Improved Leaf Pruning Reduces Development of Stem Cankers Caused by Grey Mould in Greenhouse Tomatoes.” Agronomy for Sustainable Development 30:465–472. https://doi.org/10.1051/agro/2009030
Fourtouni, A., Y. Manetas, and C. Christias. 1998. “Effects of UV-B Radiation on Growth, Pigmentation and Spore Production in the Phytopathogenic Fungus Alternaria solani.” Can J. Bot. 76:2093–2099.
Freeman, J. H., C. Frey, R. Kanissery, H. A. Smith, J. Desaeger, and G. E. Vallad. 2022. “Chapter 18. Tomato Production.” EDIS 2022 (VPH). https://doi.org/10.32473/edis-cv137-2022
Frey, C. J., X. Zhao, J. K. Brecht, D. M. Huff, and Z. E. Black. 2020. “High Tunnel and Grafting Effects on Organic Tomato Plant Disease Severity and Root-Knot Nematode Infestation in a Subtropical Climate with Sandy Soils.” HortScience 55 (1): 46–54. https://doi.org/10.21273/HORTSCI14166-19
Gill, H. K., and R. McSorley. 2017. “Cover Crops for Managing Root-Knot Nematodes.” EDIS 2011 (7). https://doi.org/10.32473/edis-in892-2011
Li, R., F. Baysal-Gurel, Z. Abdo, S. A. Miller, and K. S. Ling. 2015. “Evaluation of Disinfectants to Prevent Mechanical Transmission of Viruses and a Viroid in Greenhouse Tomato Production.” Virology Journal 12:5. https://doi.org/10.1186%2Fs12985-014-0237-5
Paudel, B. R., F. D. Gioia, Q. Zhu, X. Zhao, M. Ozores-Hampton, M. E. Swisher, K. Sattanno, J. C. Hong, and E. N. Rosskopf. 2019. “Implementation of Anaerobic Soil Disinfestation in Florida Tomato Production.” EDIS 2019 (6): 5. https://doi.org/10.32473/edis-hs1345-2019
Peres, N., G. Vallad, J. Desaeger, and H. Smith. 2021. “Chapter 19. Biopesticides and Alternative Disease and Pest Management Products.” EDIS 2022 (VPH). https://doi.org/10.32473/edis-cv295-2022
Rogers, M. A., and A. L. Wszelaki. 2012. “Influence of High Tunnel Production and Planting Date on Yield, Growth, and Early Blight Development on Organically Grown Heirloom and Hybrid Tomato.” HortTechnology 22 (4): 452–462. https://doi.org/10.21273/HORTTECH.22.4.452
Scholthof, K.-B. G. 2000. “Tobacco mosaic virus.” The Plant Health Instructor. Updated 2005. https://doi.org/10.1094/PHI-I-2000-1010-01
Simone, G. W., R. C. Hochmuth, G. C. Wisler, J. E. Duffus, H. Y. Liu, and R. H. Li. 1996. New Whitefly-Vectored Closterovirus of Tomato in Florida 96-05. UF/IFAS North Florida Research and Education Center–Suwannee Valley. https://svaec.ifas.ufl.edu/media/svaecifasufledu/docs/pdf/svreports/crops/tomato/96-05.pdf
Smith, H. A., P. A. Stansly, D. R. Seal, E. McAvoy, J. E. Polston, P. R. Gilreath, and D. J. Schuster. 2018. “Management of Whiteflies, Whitefly-Vectored Plant Virus, and Insecticide Resistance for Tomato Production in Southern Florida.” EDIS 2018 (6). https://edis.ifas.ufl.edu/publication/IN695
Smith, H. A., G. E. Vallad, and B. E. Santos. 2019. “Integrated Pest Management in Protected Structures I: Basic Principles and Scouting.” EDIS 2013 (9). https://doi.org/10.32473/edis-in994-2013
Thomma, B. P. H. J., H. P. Van Esse, P. W. Crous, and P. J. G. M. De Wit. 2005. “Cladosporium fulvum (syn. Passalora fulva), a Highly Specialized Plant Pathogen as a Model for Functional Studies on Plant Pathogenic Mycosphaerellaceae.” Molecular Plant Pathology 6 (4): 379–393. https://doi.org/10.1111/j.1364-3703.2005.00292.x
Xie, C., and G. Vallad. 2016. “Integrated Management of Southern Blight in Vegetable Production.” EDIS 2010 (3). https://doi.org/10.32473/edis-pp272-2010
Zhang, S., P. D. Roberts, R. J. McGovern, and L. E. Datnoff. 2018. “Fusarium Crown and Root Rot of Tomato in Florida.” EDIS 2021 (6). https://doi.org/10.32473/edis-pg082-2021