Gummy stem blight (GSB) is a major disease of many cucurbits, including watermelon, cantaloupe, cucumber, pumpkin, squash, muskmelon, and other melons. The fungus Didymella bryoniae is the causal organism for this disease. Infection on watermelon and cantaloupe is commonly seen in Florida, and the disease can cause significant production losses when conditions are ideal for the spread of this fungal pathogen (Figure 1). The disease is also known as black rot due to its characteristic appearance on infected fruits.
Cucurbit plants can become infected with D. bryoniae at any growth stage of the plant, from seedling to mature vine with fruit. Infection signs and symptoms can be seen on all parts of the plant except for the roots. Yellowing of the leaf margins (chlorosis) is an early symptom on the plant, and light- to dark-brown spots (necrosis) can appear on the seed leaves (cotyledons) (Figure 2). These symptoms are often visible before and after transplanting in the field. Prior to the occurrence of chlorosis or necrosis, the same tissue may appear water soaked (Figure 3). Wilting, followed by death of the transplants, can occur.
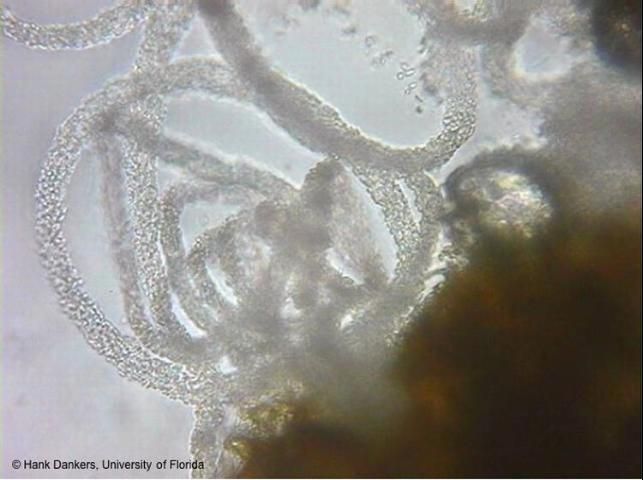
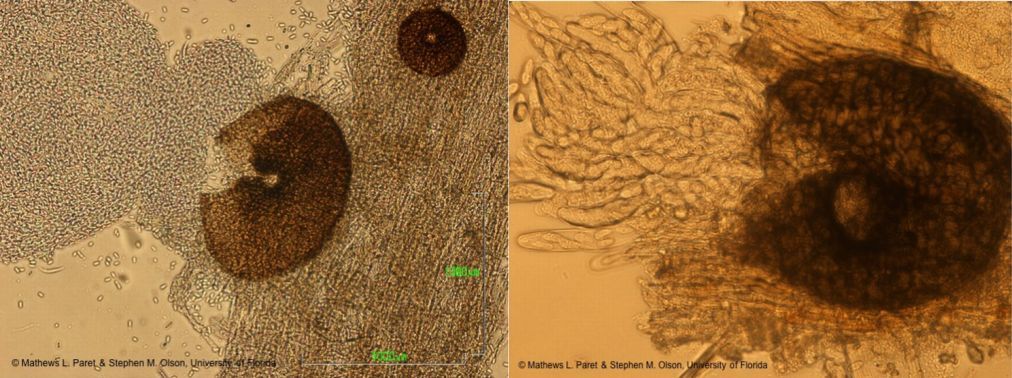
Lesions can also form on the stem that enlarge and girdle the main stem (Figure 4). Cracking is often visible on the stem, accompanied by gummy ooze (Figure 5). Cankers develop on the stem that can be red, brown, or black in color, and a red to amber gummy substance can exude from this region (Figure 6). Anthracnose (caused by Colletotrichum orbiculare) and inadequate soil liming can also cause the exudation of a gummy substance from cucurbit stems; hence, "gummyness" should not be relied upon as a definitive diagnosis of GSB. Black fruiting bodies of the fungus (pycnidia, perithecia, or pseudothecia) are often visible on the infected leaves, stems, and fruits and serve in confirmatory diagnosis (Figures 4 and 7). Pycnidia swell and release tendrils of spores if the tissue is wetted in water (Figures 8 and 9). This is easily observed with the use of a hand lens or microscope. Large, brown lesions from severe infections can form on areas of the leaves that retain moisture for long periods of time, such as around veins or leaf margins. (Figure 10). Vine wilting symptoms are usually not observed until later in the infection cycle, typically a period of three to four weeks after infection (Figure 1).
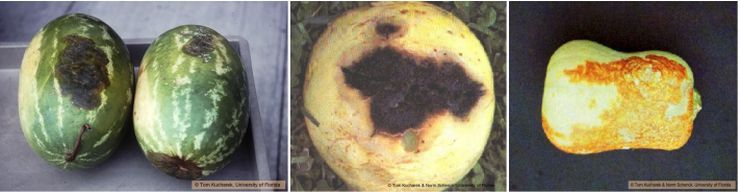
Fruit rot in watermelon is only a problem if the vines are severely infected with gummy stem blight. Lesions on fruits of cucumber, muskmelon, and watermelon are first oval to circular and greasy green in color. The lesions subsequently merge and become brownish-black in color—hence the disease is also known as black rot (Figure 11). These lesions appear depressed in the center. Internally, the rind becomes dark brown to black and cracked. Butternut squash fruit can be infected with the vines being healthy. A lesion, dark yellow to brown in color and crusty in appearance, occurs on large areas of the butternut squash (Figure 11).
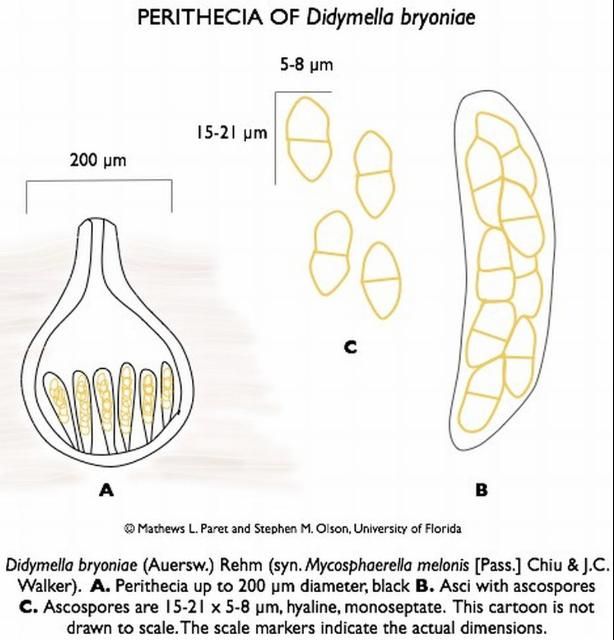
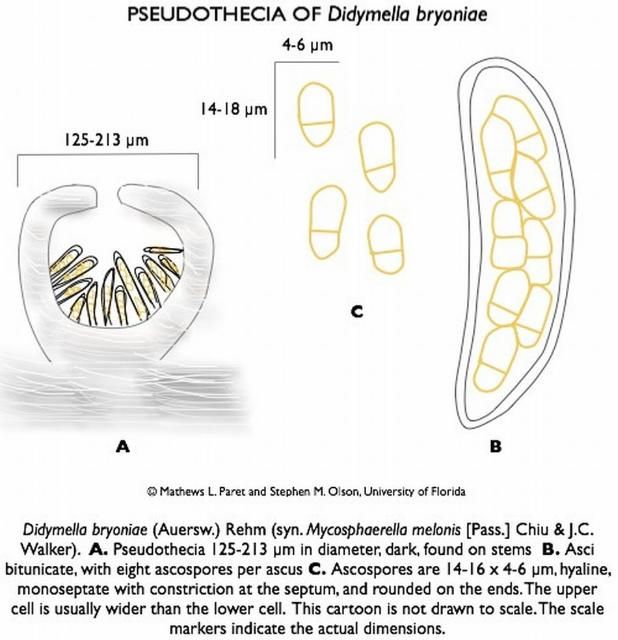
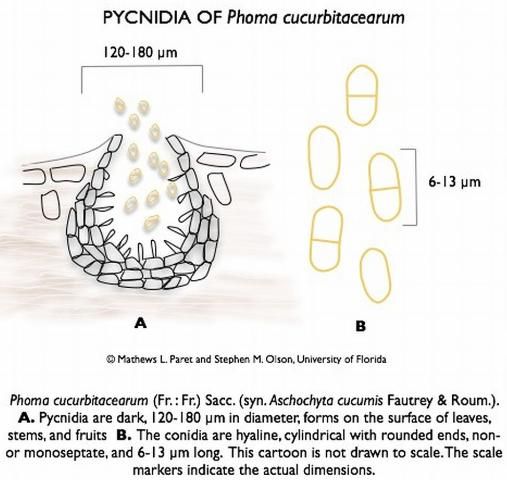
Didymella bryoniae (anamorph is Phoma cucurbitacearum) is an ascomycete fungus that can survive on seeds, weeds (citron, balsam pear, and other volunteer cucurbits), and plant debris from previously infected cucurbits. The fungus produces two spore stages, a sexually produced spore (perithecia giving rise to ascospores; Figure 12) and an asexually produced spore (pycnidia giving rise to conidia; Figure 13). Perithecia and pycnidia can be found embedded in the same lesion. Ascospores serve as the primary inoculum and are readily spread from field to field by wind. Conidia are released in a gummy substance and are therefore more adapted for short-distance movement through splashing water, which leads to secondary spread of the disease. Dark pseudothecia may also form, especially on stems (Figure 14), but are rarely seen.
Moisture and temperature play an important role in germination, sporulation, colonization of conidia into the plant tissue, and symptom development. The optimum conditions for the infection process are temperatures ranging from 61°F–75°F and a moisture level of 85% RH caused by consistent leaf wetness for 1–10 hours. Nighttime temperature and moisture conditions prevalent in Florida are ideal for GSB infection during much of the crop-growing season. After spore germination on a susceptible host tissue, symptoms can appear in 7–12 days. Wounding of host tissue by mechanical injury, feeding by aphids and striped cucumber beetles, and infections from other diseases, including powdery mildew (caused by Sphaerotheca fuliginea), may provide entry sites for the GSB pathogen. Harvest points on fruits can also be a point of entry for the pathogen, leading to postharvest decay.
A sequence of management plans must be initiated to control GSB:
-
Seed: One source of GSB inoculum is the seed. Purchase seeds from reputable companies with a good history of GSB-free seed production. Seeds from healthy fruits are free of GSB. Remember: Seeds can be infested without expressing symptoms. Seed treatment is necessary if not previously treated, and disinfectants as solutions are more effective than dry-dust treatments. GSB-resistant varieties are not available in any cucurbits.
-
Transplants: GSB is common at the seedling stage and displays one or more of the characteristic symptoms (necrotic areas on the margin of the leaves, water-soaked regions on the stem, gummy ooze from the stem). Growers should regularly inspect transplant seedlings in the greenhouses. Whenever possible, avoid using healthy-looking seedlings from trays with infected plants.
-
Organic debris: Another source of primary inoculum is organic debris from previous cucurbit crops. As soon as a cucurbit crop is harvested (especially crops with GSB inoculum), the decaying debris from that crop should be disked and deep plowed into the soil if it is to be followed by a long-term crop rotation practice.
-
Volunteer plants: Wild citrons, balsam pear, or volunteer cucurbits are other sources of inoculum and should be eradicated before planting cucurbits.
-
Crop rotation: Fields should not be cultivated with cucurbits routinely, and rotation with a non-cucurbit crop is important. A two- to three-year rotation with non-hosts is an effective way to reduce incidence of GSB.
-
Scouting: Routine scouting of the fields helps in timely application of fungicides that can prevent major crop losses.
-
Biological control and biopesticides: The effectiveness of bio-control agents currently available in the market is heavily dependent upon environmental conditions at the time of application and therefore has demonstrated minimal success against GSB.
-
Fungicide application: Apply fungicides in a preventative manner. Chlorothalonil ad Mancozeb are effective contact fungicides, and there are many effective systemic fungicides for management of GSB. If weather conditions are conducive for the occurrence and rapid spread of GSB, fungicide application should start during the early stages of plant growth. A list of currently labeled chemicals for use on cucurbits in Florida is provided in the Vegetable Production Handbook for Florida (https://edis.ifas.ufl.edu/publication/cv123). To prevent development of fungicide resistance, various chemistries should be alternated during the spray program.
-
Storage: GSB infection can also occur on the fruit. Avoid wounding fruits during harvest, and store fruits at 45°F–50°F to prevent postharvest black rot.
Ellis, M. B., and J. P. Ellis. 1985. Microfungi on Land Plants: An Identification Handbook. New York: Macmillan.
Dufault, N. S., and M. L. Paret. 2017. Watermelon spray guide for Florida. UF/IFAS. http://plantpath.ifas.ufl.edu/u-scout/Tutor.html
Freeman, J. H., E. J. McAvoy., N. S. Boyd., M. Ozores-Hampton., M. Paret., Q. Wang., C. F. Miller., J. W. Noling., X. Martini. 2017. "Cucurbit Production", In: Vegetable Production Handbook for Florida, edited by G. E. Vallad., H. A. Smith., P. J. Dittmar., J. H. Freeman.
Sitterly, W. R., and A. P. Keinath. 1996. "Gummy Stem Blight." Compendium of Cucurbit Diseases, edited by T. A. Zitter, D. L. Hopkins, and C. E. Thomas, 27–28. St. Paul, MN: American Phytopathological Society.
Zitter, T.A. 1992. Gummy Stem Blight. Vegetable MD Online Fact Sheet. Ithaca, NY: Cornell University. https://hdl.handle.net/1813/43278