Introduction
Watermelon (Citrullus lanatus), cantaloupe or muskmelon (Cucumis melo), squash (Cucurbita pepo), bitter gourd (Momordica charantia), and cucumber (Cucumis sativa) are commonly grown throughout Florida on both commercial farms and home gardens, while pumpkin (Cucurbita maxima) production is largely limited to north Florida. Anthracnose on cucurbits, caused by the fungus Colletotrichum orbiculare, is a common disease that occurs worldwide and throughout Florida. While all cucurbits are susceptible to the disease, cucumber, cantaloupe, and watermelon are most frequently affected.
Historically, anthracnose has occurred more frequently on cucurbits—mainly on cantaloupe and increasingly on watermelon—grown in north Florida but, more recently, has been of growing importance in south Florida. Severe losses can occur on susceptible hosts by defoliation caused by lesions on leaves, peduncles, and stems and from lesions on fruit that make them non-marketable.
Causal Organism
Anthracnose is caused by the fungal pathogen Colletotrichum orbiculare. Colletotrichum species are ubiquitous, causing diseases of significance on many crops throughout the world. Most have a wide host range, such as C. orbiculare, which has been reported on other hosts including many weeds (Damm et al. 2013). On cucurbits, there are three races (1, 2, and 2B) of C. orbiculare that exhibit some host specificity and are characterized based on a set of differential hosts including watermelon and cucumber (Wasilwa et al. 1993). Race 1 is pathogenic on cucumber; race 2 isolates are pathogenic on watermelon. And race 2B is intermediate depending upon the host (watermelon or cucumber). Other cucurbits show variable responses to the three races. More recently, there are reports from other cucurbit production regions that multiple species of Colletotrichum cause anthracnose on cucurbits (Guo et al. 2022).Symptoms and Signs
The fungus infects all above-ground plant tissues, including the leaves, stems, peduncles, and fruit. It is seedborne, and seedling symptoms appear as dark brown lesions on the cotyledons. Although symptoms of anthracnose can vary in appearance depending on the host, in general, lesions on leaves start as water-soaked areas that become tan to dark brown and are roughly circular or sometimes angular in appearance (Figures 1–4). In severe infections, lesions can coalesce, and the affected leaf area appears blighted. Stem lesions are elongated. Lesions on fruit can also have slightly different appearances depending upon the host but are generally mostly circular with a salmon-colored appearance due to the masses of spores on the lesion surface (Figures 5 and 6).

Credit: K. Hendricks, UF/IFAS

Credit: U-Scout/M. Paret, UF/IFAS

Credit: U-Scout/H. Dankers, UF/IFAS

Credit: Shouan Zhang, UF/IFAS

Credit: K. Hendricks, UF/IFAS

Credit: U-Scout/H. Dankers, UF/IFAS
Identification of the fruiting bodies and spores is diagnostic for this disease. Dark acervuli (fruiting bodies) and a profusion of single-celled spores (conidia) are visible with a microscope (Figures 7 and 8). Additionally, the salmon-colored lesions are indicative of anthracnose (Figure 9).

Credit: U-Scout/H. Dankers, UF/IFAS
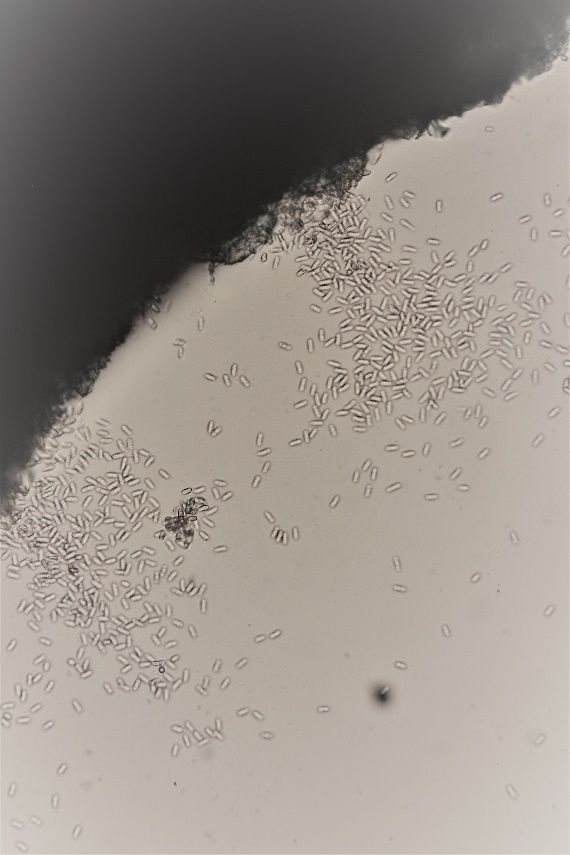
Credit: K. Hendricks, UF/IFAS

Credit: U-Scout/H. Dankers, UF/IFAS
Disease Cycle
The fungus is seedborne, and this can be a primary inoculum source. Studies have found that the fungus can survive for up to 2 years in the soil in the absence of a host. Warm, wet conditions favor the development of anthracnose, and the most severe outbreaks are associated with frequent rain events. The spores are dispersed by water such as rain events or heavy dew. Spores can be carried by people and machinery moving through wet plants in the field. Fruit can be infected while still in the field, but post-harvest fruit infection can happen if fruit comes into contact with infected fruit during harvesting or shipping or are washed in water containing spores from infected fruit.
The optimum temperature range for infection on watermelon was shown to be 70°F to 75°F (Monroe et al. 1997) while the temperature range for cucumber infection ranged from 68°F to 82°F with the greatest conidia production at 75°F. Long periods of leaf wetness, more than 16 hours, lead to greater disease development (Monroe et al. 1997; Thompson and Jenkins 1985).
Integrated Management
An integrated pest management program is recommended for anthracnose control. Whenever possible, use anthracnose-resistant varieties and disease-free transplants. Plants should be scouted frequently for disease symptoms. At the end of the season, remove or incorporate plant debris and do not replant susceptible cucurbits in the same field for at least a year. Cucurbit volunteers and alternative hosts in and around the field and transplant house should be eliminated. Avoid moving machinery or workers in the fields when the foliage is wet.
Chemical Control
Current, labeled fungicides are presented in chapter 7, “Cucurbit Production,” in Vegetable Production Handbook of Florida available at HS725/CV123: Chapter 7. Cucurbit Production (ufl.edu). It is reported in other production regions that the fungal population of anthracnose is becoming insensitive to the most widely labeled and used active ingredient group, the strobilurins, a quinone outside inhibitor (QoI) categorized by the Fungicide Resistance Action Committee (FRAC) under group 11 of their FRAC Code. Field observations on cucurbits in Florida tend to support this; therefore, until more information is available, strobilurin fungicides should be used early, with caution, and adhering to labeled rotational guidelines. Fungicide applications should be made at the first appearance of the disease and continued at regular intervals as long as favorable conditions exist (moderate weather, wet foliage). Rotation of fungicides following resistance management guidelines should be followed. A current list of biological pesticides and other materials can be found in chapter 19 (CV295/CV295: Chapter 19. Biopesticides and Alternative Disease and Pest Management Products (ufl.edu). These products are best used with other integrated management techniques.
References
Damm, U.; P. F. Cannon, F. Liu, R. W. Barreto, E. Guatimosim, and P. W. Crous. 2013. “The Colletotrichum orbiculare Species Complex: Important Pathogens of Field Crops and Weeds.” Fungal Diversity 61: 29–59. https://doi.org/10.1007/s13225-013-0255-4
Guo, Z., C. X. Luo, H. J. Wu, B. Peng, B. S. Kang, L. M. Liu, M. Zhang, and Q. S. Gu. 2022. “Colletotrichum Species Associated with Anthracnose Disease of Watermelon (Citrullus lanatus) in China.” Journal of Fungi 8, no. 8: 790. https://doi.org/10.3390/jof8080790
Keinath, A.P. 2017. “Anthracnose.” In Compendium of Cucurbit Diseases and Pests, edited by A.P. Keinath, W. M. Wintermantel, and T. A. Zitter, 54-55. Saint Paul: APS Press. https://doi.org/10.1094/9780890545744
Monroe J. S., J. B. Santini, and R. Latin. 1997. “A Model Defining the Relationship between Temperature and Leaf Wetness and Infection of Watermelon by Colletotrichum orbiculare.” Plant Disease 81 (7):739–742. https://doi.org/10.1094/PDIS.1997.81.7.739
Thompson, D. C. and S. F. Jenkins. 1985. “Effect of Temperature, Moisture, and Cucumber Cultivar Resistance on Lesion Size Increase and Conidial Production by Colletotrichum lagenarium.” Phytopathology 75 (7):828–832. https://doi.org/10.1094/Phyto-75-828
UF/IFAS. U-scout. Cucurbit diseases. https://plantpath.ifas.ufl.edu/u-scout/cucurbit/index.html
Wasilwa, L. A., J. C. Correll, T. E. Morelock, and R. E. McNew. 1993. “Reexamination of Races of the Cucurbit Anthracnose Pathogen Colletotrichum orbiculare.” Phytopathology 83 (11):1190-1198. https://doi.org/10.1094/Phyto-83-1190